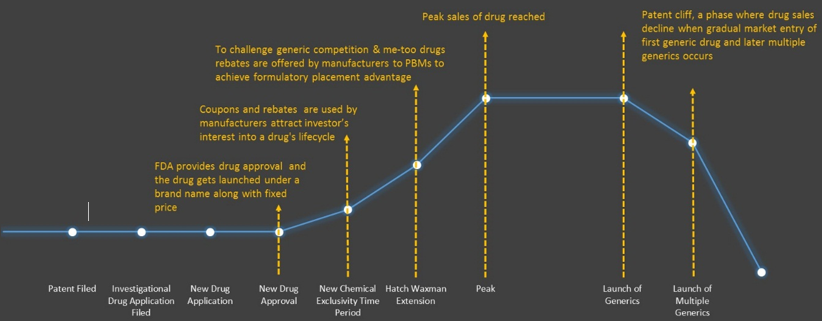
We understand the importance of drug lifecycle and would recommend strategies which will assist a company to increase the longevity of drug’s existence in particular market. The competition is getting fierce in autoimmune diseases & oncology treatment market but on the similar basis it has been observed in several other diseases. Our report covers this section where market experts and our in-house analysts provide robust strategies which would help a company to manage drug lifecycle in any market. We also provide brief description about the recommended strategies specifically targeting clinical trials or consumer marketing. Our report provides consultation for four major levels of drug lifecycle which can ultimately assist in longevity and management of drug’s optimum commercial performance.
The four major levels of drug lifecycle that are targeted by Credence Research are as follows:
- Pre-clinical Stage of Research & Development
- Clinical Trials
- Drug Approval
- Product Launch, Marketing & Penetration
There are several strategies for extending the life cycle of drugs, to depend on simple developments of current technologies is becoming challenging. Governments, medical practitioners, payers, and patients frequently demand evidence-based drugs and anticipate novel therapies to either deliver highly improved results or meet the unmet medical needs. To provide an exhaustive life cycle management strategies with such complex conditions, we extract & collect inputs from several market stakeholders. A cross-functional response team is interviewed while providing this service that comprises key persons working in marketing, research & development, production, regulatory, process engineering and others across targeted therapeutic areas. Such a universal or cohesive approach results in the search & identification of novel markets and latest technologies for prolonging the lifetime of drugs.