Credence Research is an independent service provider that offers a comprehensive portfolio of a regulatory framework, life science consulting, clinical research solutions, R&D technology, medical informatics and compliance services to help solve complex challenges. Our mission is to improve patient health and safety, and we focus on delivering the highest quality services throughout the product lifecycle. Our customers include API manufacturers, manufacturers of various finished dosage forms, contract manufacturing organizations (CMOs), intermediate processors, testing facilities, medical device manufacturers, homeopaths and food ingredient suppliers.
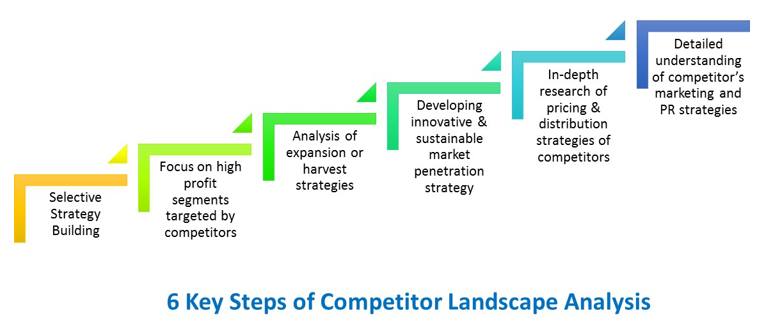
Identifying and evaluating competition is critical to corporate strategic planning to gain and maintain market share. We provide comprehensive support to give our clients a competitive advantage. The issues we address in competitive insights are company profiles of major key players, including their financial performance, products/services offered, distribution channels, and strategic initiatives. We also address industry best practices and strategic recommendations related to R&D-based markets (chemical or biological), vaccines markets – generics and biosimilar markets, OTCs, dietary supplements, cosmeceuticals, and medical device markets. Additionally, we provide SWOT, PESTEL and Porter’s analysis to evaluate competitive threats, identification of merger, acquisition and partnership targets, and competitor benchmarking for performance measurement. We validate our assumptions by conducting regular interviews with companies for benchmarking studies, physicians such as KOLs, specialists & GPs, distributors (wholesalers, agents, purchasing groups, retail and hospital pharmacists, etc.), health authorities, payers, patients and Patient Advocacy Groups (PAGs), etc.
Early Concept Development
Our experienced team helps you address complex regulatory and compliance challenges by developing strategies for successful regulatory and commercialization outcomes. As a partner, we work with you to ensure success in the early concept stages of the product development lifecycle.
Clinical Development Support
With in-depth knowledge of client processes, our team of experts quickly identifies and resolves quality or compliance issues while complying with local regulations worldwide. We provide a full suite of compliance and clinical drug safety services to advance your product through clinical development efficiently.
Product Postmarketing
Our end-to-end service suite includes support for your postmarketing requirements to ensure the quality and safety of your products. We provide drug safety and compliance services with bilingual experts using integrated technologies to deliver regionally-focused solutions that can scale globally based on client needs.
We have earned a well-deserved reputation for trust, expertise, and highly successful project outcomes. We believe that our years of experience and hard-working expertise equals positive results.
Regulatory Science Consulting Services
Our regulatory consultants focus on pharmaceutical, medical device, diagnostic development, and regulatory aspects.
Using our novel approach combining deep scientific knowledge and extensive regulatory experience, our drug regulatory experts help clients achieve successful outcomes within the FDA and EMA with regulators on every continent.
From the early development of your product to approval and commercialization, our EMA and FDA compliance consultants can assist you with all your regulatory needs throughout the product lifecycle.
Life Science Consulting Services
Our team of qualified life science consultants employs proven and patented project management methodologies to serve our clients and strengthen their product portfolio. Credence Research provides a complete suite of life science consulting services to ensure quality, efficiency, compliance, and safety during the product lifecycle. Working with your team experts, our life science consultants provide you with the highest quality of service by offering best practices and proven insights on the process and program management aligned with our mission to improve patients’ health and safety.
Clinical Research Solutions
Our Clinical Research Solutions team continually assesses enforcement trends and invests in industry expertise to lead our clients to compliance and operational stability. Our team of clinical research experts brings leadership to your project and can help you complete important and complex projects throughout the clinical development process. We execute your most complex and resource-dependent programs. We have trusted advisors with an extensive track record in developing effective clinical research solutions with far-reaching impact.
R&D Technology
Our team has system experience throughout the product life cycle, so you don’t have to work with a different team for each system. We handle your technical projects using a proven framework for efficient requirements gathering and fit-for-purpose service delivery. Our team understands FDA, EMA, MHRA, and PMDA regulations and can help ensure compliance.
Expert Medical Information Solutions
With strategically positioned and seamlessly integrated contact centers worldwide, we provide customizable healthcare information services to meet the needs of companies of all sizes. Whether you need adverse event receipt and follow-up, medical writing and content management solutions, promotional reviews, or other extended medical information services, our medical information specialists have the knowledge and experience to support your needs effectively.
Get In Touch With Our Team On How We Can Help You