Market Overview:
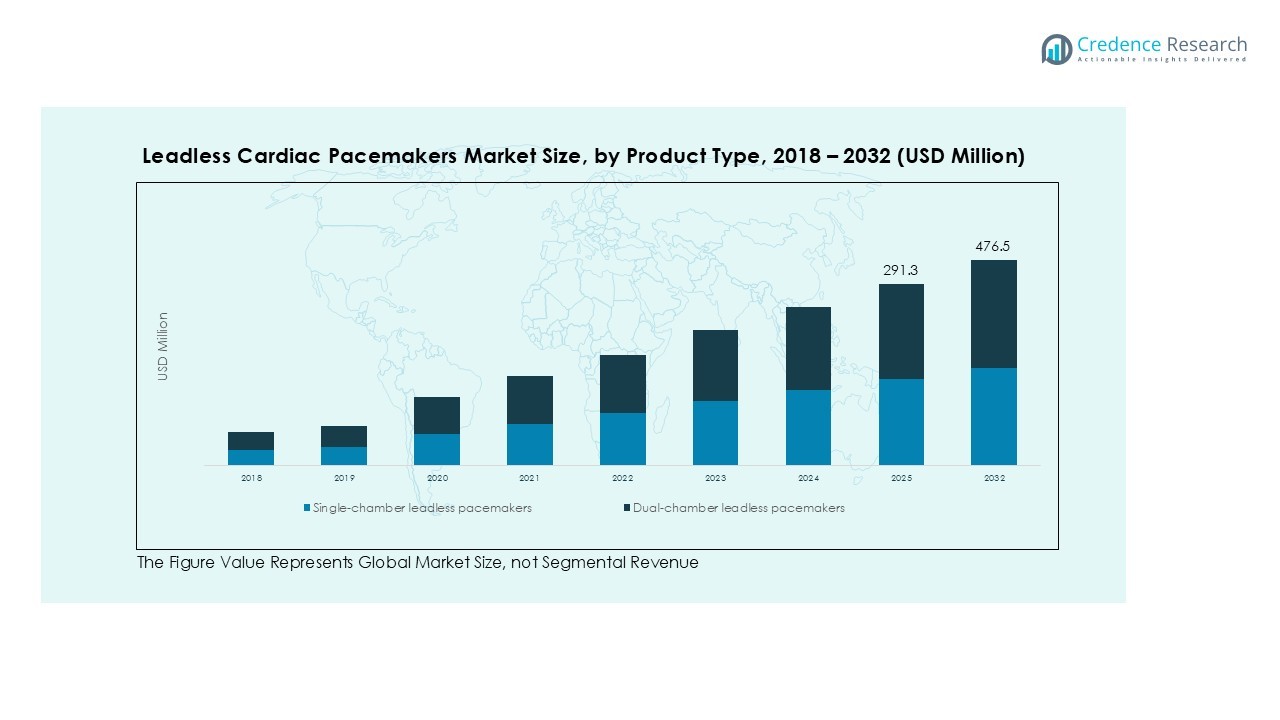
The Global Leadless Cardiac Pacemakers Market size was valued at USD 220.00 million in 2018 to USD 273.68 million in 2024 and is anticipated to reach USD 476.48 million by 2032, at a CAGR of 7.28% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Leadless Cardiac Pacemakers Market Size 2024 |
USD 273.68 Million |
| Leadless Cardiac Pacemakers Market, CAGR |
7.28% |
| Leadless Cardiac Pacemakers Market Size 2032 |
USD 476.48 Million |
Market expansion is primarily influenced by the rising prevalence of cardiac arrhythmias and increasing geriatric populations globally. The growing demand for minimally invasive cardiac procedures is fostering greater acceptance of leadless pacemakers, which eliminate lead-related complications and lower infection risks. Moreover, supportive government initiatives for advanced cardiac care and favorable reimbursement policies in developed economies are propelling market penetration.
Regionally, North America holds the largest share of the Leadless Cardiac Pacemakers Market, supported by advanced healthcare infrastructure and high adoption of innovative cardiac devices. Europe follows closely due to a well-established clinical framework and strong emphasis on patient safety. The Asia-Pacific region is poised for the fastest growth, driven by rising cardiovascular disease incidence, expanding healthcare access, and increasing investments in medical technology infrastructure.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights:
- The Global Leadless Cardiac Pacemakers Market was valued at USD 273.68 million in 2024 and is projected to reach USD 476.48 million by 2032, registering a CAGR of 7.28% during the forecast period.
- Rising incidences of cardiac arrhythmias and an expanding geriatric population worldwide are major factors fueling market demand for safer and more efficient pacing solutions.
- The shift toward minimally invasive cardiac procedures continues to accelerate market adoption, as leadless pacemakers eliminate surgical leads and significantly reduce infection risks.
- Technological advancements, including device miniaturization, extended battery life, and wireless connectivity, enhance performance and expand clinical acceptance across healthcare institutions.
- North America dominates the market with a 40% share, supported by advanced healthcare infrastructure, favorable reimbursement frameworks, and high patient awareness.
- The Asia-Pacific region is projected to witness the fastest growth with a CAGR of 9.3%, driven by rising cardiovascular disease prevalence, expanding healthcare access, and medical technology investments.
- High device costs, limited reimbursement in emerging economies, and the need for specialized physician training remain key challenges, though growing innovation and supportive health policies continue to strengthen long-term market potential.
Market Drivers:
Rising Burden of Cardiac Arrhythmias and Aging Population
The growing prevalence of cardiac arrhythmias such as atrial fibrillation and bradycardia significantly drives the Global Leadless Cardiac Pacemakers Market. It benefits from an aging global population that faces higher susceptibility to heart rhythm disorders, requiring advanced pacing solutions. The World Health Organization reports a consistent increase in cardiovascular disease incidence, creating a strong demand base for compact and efficient cardiac devices. Leadless pacemakers provide improved clinical outcomes by minimizing surgical complications and enhancing patient safety. This demographic and epidemiological shift continues to sustain long-term market growth.
- For instance, Medtronic’s Micra VR leadless pacemaker demonstrated a 99.2% implant success rate in over 700 patients, indicating significantly reduced surgical complications compared to conventional transvenous pacemakers.
Shift Toward Minimally Invasive Cardiac Procedures
Healthcare providers increasingly prefer minimally invasive approaches to reduce recovery time, infection risk, and hospital stay duration. The Global Leadless Cardiac Pacemakers Market gains traction through this trend, as these devices eliminate leads and surgical pockets. It supports patient comfort while improving procedural efficiency and lowering device-related complications. Hospitals and cardiac centers are expanding their use of leadless technology to meet modern standards of precision and safety. The shift aligns with healthcare systems’ focus on better outcomes and cost optimization.
- For Instance, Abbott accomplished a milestone with its AVEIR DR dual-chamber leadless pacing system achieving 98.3% successful implantation rate with implant-to-implant communication and demonstrating 88.6% freedom from system-related complications at 12 months.
Technological Advancements Enhancing Device Performance
Continuous innovation in pacemaker technology significantly strengthens market adoption. It includes extended battery longevity, advanced sensing algorithms, and improved wireless communication capabilities. These developments enhance clinical reliability and reduce the need for repeated interventions. The Global Leadless Cardiac Pacemakers Market benefits from ongoing research efforts that refine device miniaturization and improve procedural guidance. Enhanced device functionality promotes physician confidence and broadens usage across diverse patient profiles.
Supportive Healthcare Policies and Reimbursement Structures
Government policies and insurance coverage play a vital role in encouraging leadless pacemaker implantation. The Global Leadless Cardiac Pacemakers Market experiences growth due to favorable reimbursement in developed regions and increasing healthcare funding in emerging economies. It gains further momentum from regulatory approvals that expand product availability and accelerate market entry. Healthcare authorities prioritize advanced cardiac care solutions that ensure patient safety and reduce lifetime healthcare costs. These policy measures continue to create a stable environment for sustained market expansion.
Market Trends:
Expansion of Dual-Chamber and Atrioventricular Synchronization Capabilities
Manufacturers introduce advanced dual-chamber leadless pacemakers to offer atrial plus ventricular pacing without leads. It delivers better physiological pacing, improving cardiac output in patients who need more complex rhythm support. Clinical trials validate performance and safety of newer dual-chamber systems, driving regulatory approvals in several markets. The transition from single-chamber to dual-chamber systems broadens the patient pool. It encourages investment in device design that maintains minimal invasiveness while adding functionality. Physicians show rising interest in devices that balance synchrony, durability, and patient comfort.
- For Instance, Medtronic’s Micra AV2 is expected to achieve over 98% successful implantation rates with procedural times often under 30 minutes, consistent with results for previous Micra models
Integration of Remote Monitoring, Predictive Analytics, and Wireless Connectivity
Remote patient monitoring gains prominence in postoperative care and long-term device performance tracking. Leadless pacemakers with built-in telemetry send data on battery status, heart rhythms, and pacing performance to care teams. It improves follow-up efficiency, reduces hospital visits, and supports early detection of complications. Predictive algorithms analyze trends to adjust device behavior or alert clinicians before adverse events occur. Regulatory bodies recognize these smart features during approval processes, particularly for devices offering wireless connectivity and enhanced safety. Market players embed digital health capabilities into product pipelines to meet demand for more connected, data-driven cardiac care.
- For Instance, Boston Scientific’s HeartLogic™ diagnostic, delivered via the LATITUDE™ platform, uses predictive analytics to identify worsening heart failure, providing a median of 34 days advance notice of a potential event.
Market Challenges Analysis:
High Device Cost and Limited Reimbursement Accessibility
The premium pricing of leadless pacemakers continues to be a major restraint within the Global Leadless Cardiac Pacemakers Market. It restricts adoption across developing regions where healthcare budgets and reimbursement support remain limited. Hospitals face challenges in justifying high upfront costs despite long-term clinical benefits. Limited insurance coverage for innovative cardiac implants further slows penetration in middle-income economies. Healthcare systems that prioritize cost containment often favor traditional pacing devices. This financial barrier impacts patient accessibility and slows overall market expansion despite growing clinical acceptance.
Technical Complexity and Limited Physician Familiarity
The implantation of leadless pacemakers requires specialized procedural training and advanced imaging support. The Global Leadless Cardiac Pacemakers Market encounters challenges due to limited physician experience and the steep learning curve associated with device deployment. It raises procedural risks during early adoption phases, particularly in centers without experienced cardiac electrophysiologists. Retrieval difficulties and device repositioning constraints also concern healthcare professionals. Regulatory delays related to product safety evaluations extend approval timelines for new entrants. These technical and operational barriers collectively limit rapid global scalability despite strong technological progress.
Market Opportunities:
Growing Adoption Across Emerging Healthcare Markets
Expanding healthcare infrastructure and increased cardiac care investment in developing economies create strong opportunities for the Global Leadless Cardiac Pacemakers Market. It benefits from government initiatives aimed at modernizing cardiac treatment facilities and improving access to advanced medical technologies. Rising awareness among clinicians and patients about the benefits of leadless systems supports demand growth. Collaborations between global manufacturers and regional distributors improve device availability and affordability. Hospitals in Asia-Pacific, Latin America, and the Middle East are increasingly integrating leadless pacing into cardiac treatment protocols. These developments open new revenue channels and diversify market presence beyond mature economies.
Innovation in Device Design and Multi-Chamber Capabilities
Technological progress in miniaturization and multi-chamber pacing creates significant growth potential for industry players. The Global Leadless Cardiac Pacemakers Market gains momentum from research focused on atrioventricular synchronization and improved power efficiency. It encourages manufacturers to develop next-generation systems with longer battery life and enhanced communication features. Integration of wireless monitoring and AI-based diagnostics enhances patient management and long-term care outcomes. Strategic partnerships between device manufacturers and healthcare providers accelerate clinical adoption. These advancements position leadless pacemakers as a key component of the evolving digital cardiac care ecosystem.
Market Segmentation Analysis:
By Product Type
The Global Leadless Cardiac Pacemakers Market includes single-chamber and dual-chamber leadless pacemakers. Single-chamber devices dominate due to simpler implantation procedures and suitability for patients requiring isolated atrial or ventricular pacing. Dual-chamber devices gain traction for patients needing synchronized atrioventricular pacing, improving cardiac efficiency and clinical outcomes.
- For Instance, Abbott’s AVEIR DR dual-chamber system achieved a 98.3% successful implantation rate in a clinical trial involving 300 patients.
By Application
The market serves bradyarrhythmia, atrial fibrillation, heart failure, and other cardiac conditions. Bradyarrhythmia holds the largest share because of the high prevalence of AV block and sinus node dysfunction among aging populations. Atrial fibrillation and heart failure applications are expanding, driven by minimally invasive solutions that reduce lead-related complications and infection risks.
- For instance, Boston Scientific’s INGEVITY+ pacing leads demonstrated 95.6% implant success with left bundle or left septal capture and achieved mean pacing capture thresholds of 0.94V at 0.4 ms at 3 months for left bundle branch area applications.
By End-User
Hospitals and inpatient facilities form the primary segment, supported by advanced surgical infrastructure and trained electrophysiologists. Cardiac centers and ambulatory surgical facilities contribute significantly, supported by patient preference for minimally invasive procedures and shorter recovery times. Outpatient clinics, while smaller in share, show growth potential through specialized cardiac programs and improved access to device implantation.
Segmentations:
By Product Type:
- Single-chamber leadless pacemakers
- Dual-chamber leadless pacemakers
By Application:
- Bradyarrhythmia (AV block, sinus node dysfunction)
- Atrial Fibrillation
- Heart Failure
- Others
By End-user:
- Hospitals / Inpatient Facilities
- Ambulatory Surgical Centers
- Cardiac Centers
- Outpatient Clinics
- Others
By Region:
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
North America
North America holds the largest share of the Leadless Cardiac Pacemakers Market, accounting for 40% of total revenue in 2024, valued at USD 141.93 million and projected to reach USD 246.87 million by 2032 at a CAGR of 7.3%. It benefits from advanced healthcare infrastructure, high adoption of innovative cardiac technologies, and favorable reimbursement frameworks. Rising prevalence of cardiac arrhythmias among aging populations drives strong demand. Leading manufacturers prioritize North America for product launches due to efficient regulatory approvals. Hospitals and specialized cardiac centers provide skilled electrophysiologists and advanced procedural support. It maintains market leadership through continuous technological advancements and clinical research initiatives.
Europe
Europe represents 25% of the market, growing from USD 53.66 million in 2024 to USD 86.75 million by 2032 at a CAGR of 6.3%. It benefits from well-established clinical frameworks and emphasis on patient safety. Increasing elderly populations and demand for minimally invasive procedures accelerate adoption. Strong government support and healthcare funding enhance accessibility to advanced cardiac care. Germany, France, and the U.K. remain key contributors due to high procedural volumes and hospital infrastructure. It experiences steady growth through collaborations with device manufacturers and regulatory alignment under EU medical directives.
Asia Pacific
Asia Pacific accounts for 20% of the market and is projected to expand at the fastest CAGR of 9.3%, rising from USD 44.73 million in 2024 to USD 90.48 million by 2032. It benefits from rising cardiovascular disease incidence and improvements in healthcare access. Investments in medical technology infrastructure in China, Japan, and India support market expansion. Growing clinical awareness and patient preference for minimally invasive devices strengthen adoption. Government initiatives focus on upgrading cardiac care facilities. It continues to present strong growth potential through cost-effective innovations and increasing hospital capacity.
Latin America
Latin America holds 7% of the market, increasing from USD 14.23 million in 2024 to USD 22.62 million by 2032 at a CAGR of 6.1%. It gains momentum from improved healthcare infrastructure, growing awareness of heart rhythm disorders, and modernization of medical facilities. Brazil and Mexico lead the region with expanding hospital networks and specialized cardiac centers. It benefits from collaborations between local distributors and global manufacturers to improve device availability. Gradual adoption of minimally invasive procedures supports steady growth. Public health initiatives promote cardiac screening and device implantation programs.
Middle East
The Middle East represents 5% of the market, valued at USD 11.71 million in 2024 and projected to reach USD 18.73 million by 2032 at a CAGR of 6.2%. It experiences growth from increasing cardiac disease prevalence and rising healthcare expenditures. Hospitals in the Gulf Cooperation Council countries integrate leadless pacemakers into standard cardiac care. Government support for advanced cardiac treatment and physician training programs improves device adoption. Investments in hospital infrastructure enhance procedural efficiency. It benefits from rising clinical awareness and expanding patient access to innovative cardiac technologies.
Africa
Africa contributes 3% of the market, growing from USD 7.42 million in 2024 to USD 11.04 million by 2032 at a CAGR of 4.7%. It faces challenges from limited healthcare infrastructure and economic constraints. South Africa leads regional adoption due to better hospital resources and availability of cardiac specialists. It gains traction from public health programs and international medical partnerships promoting minimally invasive cardiac treatments. Gradual improvements in healthcare training and funding support steady market expansion. Awareness campaigns and pilot programs encourage broader device utilization across urban healthcare centers.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- Medtronic plc
- Abbott Laboratories
- Boston Scientific Corporation
- EBR Systems, Inc.
- BIOTRONIK SE & Co. KG
- MicroPort Scientific Corporation
- Lepu Medical (Beijing) Co., Ltd.
- Sorin Group
- LivaNova PLC
Competitive Analysis:
The Global Leadless Cardiac Pacemakers Market demonstrates a highly competitive landscape dominated by key players focusing on technological innovation, strategic partnerships, and regional expansion. It experiences intense rivalry among established manufacturers such as Medtronic, Abbott Laboratories, and Boston Scientific, who continually enhance device performance, miniaturization, and battery longevity to maintain market leadership. Companies pursue mergers and acquisitions to broaden their product portfolios and strengthen global presence, while new product launches target unmet clinical needs in minimally invasive cardiac care. Regional expansion into emerging markets, including Asia Pacific and Latin America, allows players to capitalize on rising cardiovascular disease prevalence and growing healthcare infrastructure. Strategic collaborations with hospitals, research institutions, and distributors further improve market penetration and customer access. The competitive environment drives continuous innovation, cost optimization, and regulatory compliance, ensuring that it remains responsive to evolving clinical requirements and patient safety standards.
Recent Developments:
- In October 2025, Medtronic announced that its BrainSense™ Adaptive Deep Brain Stimulation technology was named a 2025 TIME Best Invention, following FDA and CE approvals and recent pivotal trial results.
- In September 2025, Boston Scientific unveiled plans for a next-generation Watchman device, targeting a launch in the second half of 2027 or early 2028, with ongoing trials and label expansion for the Watchman FLX Pro.
Report Coverage:
The research report offers an in-depth analysis based on Product Type, Application, End-User and Region. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook:
- Increasing prevalence of cardiac arrhythmias and aging populations will continue to drive demand for leadless pacemakers.
- Growing adoption of minimally invasive procedures will enhance patient safety and procedural efficiency.
- Technological innovations in device miniaturization, battery longevity, and wireless communication will expand clinical applications.
- Expansion into emerging markets will create opportunities for broader patient access and regional growth.
- Strategic collaborations between manufacturers, hospitals, and research institutions will accelerate product adoption and clinical trials.
- Government support and favorable reimbursement policies in developed regions will encourage wider implantation of leadless devices.
- Integration of remote monitoring and telemedicine capabilities will improve post-implant care and follow-up efficiency.
- Increasing awareness among physicians and patients regarding lead-related complications will support market preference for leadless solutions.
- Investments in healthcare infrastructure and training programs will facilitate procedural adoption in regions with limited specialist availability.
- Continuous focus on improving device safety, reliability, and cost-effectiveness will strengthen long-term market sustainability and clinical acceptance.






