Market Overview:
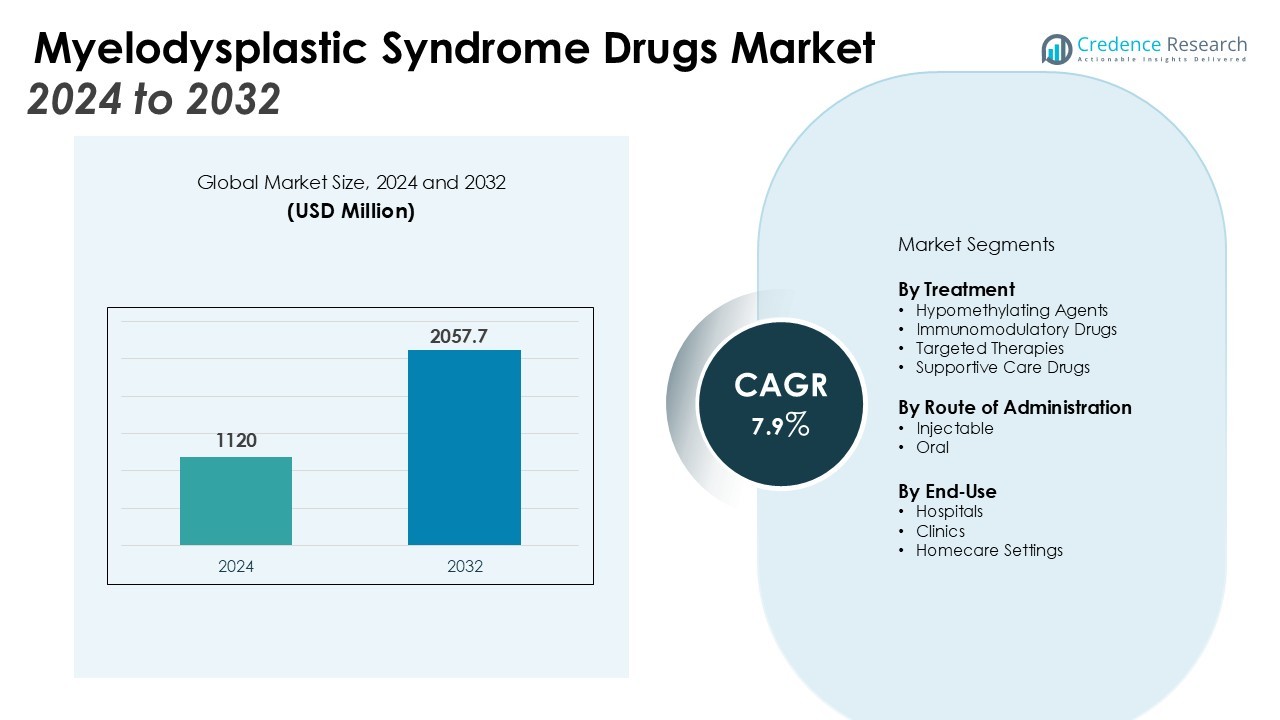
The Myelodysplastic Syndrome Drugs Market size was valued at USD 1120 million in 2024 and is anticipated to reach USD 2057.7 million by 2032, at a CAGR of 7.9% during the forecast period (2024-2032).
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Myelodysplastic Syndrome Drugs Market Size 2024 |
USD 1120 Million |
| Myelodysplastic Syndrome Drugs Market, CAGR |
7.9% |
| Myelodysplastic Syndrome Drugs Market Size 2032 |
USD 2057.7 Million |
The market is primarily fueled by the rising geriatric population, as myelodysplastic syndrome is more common in older adults. Ongoing innovation in hypomethylating agents, targeted therapies, and immunotherapies is improving treatment outcomes and widening available options for patients. At the same time, greater disease awareness combined with progress in early diagnosis is encouraging broader treatment adoption and patient access to care. Increasing investment in research and development is further strengthening the pipeline of advanced treatment solutions.
North America maintains a leading position, supported by robust healthcare infrastructure and consistent regulatory approvals that accelerate drug uptake. Europe also contributes a significant share of revenue, benefiting from structured clinical practices and the presence of a large elderly population. The Asia Pacific region is expected to experience the fastest expansion, with improving healthcare systems, growing patient awareness, and an increasing number of clinical trials driving opportunities for both existing and emerging therapies.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights:
- The Myelodysplastic Syndrome Drugs Market was valued at USD 1120 million in 2024 and is projected to reach USD 2057.7 million by 2032 at a CAGR of 7.9%.
- Rising geriatric populations remain a key driver, as the condition is most common in individuals over 60.
- Innovation in hypomethylating agents, targeted therapies, and immunotherapies is expanding treatment choices and improving patient outcomes.
- Growing awareness and earlier diagnosis are strengthening treatment adoption and enabling timely medical intervention.
- High treatment costs and limited accessibility, especially in low and middle-income regions, remain critical challenges.
- North America holds 35% of global revenue, supported by advanced infrastructure and favorable reimbursement systems, while Europe follows with 28% share driven by structured care and strong academic collaborations.
- Asia Pacific captured 22% of revenue and is the fastest-growing region, driven by rising clinical trials, improving healthcare systems, and a large underserved patient pool.
Market Drivers:
Rising Geriatric Population Driving Demand
The Myelodysplastic Syndrome Drugs Market is strongly influenced by the rapid growth of the elderly population worldwide. The condition is more prevalent among individuals over 60, which directly expands the patient pool requiring effective treatment options. With life expectancy increasing and healthcare systems identifying more cases, the demand for reliable therapies continues to grow. It is clear that demographic shifts are a critical driver, strengthening the long-term outlook for this market.
- For instance, in the United States, where the average age of diagnosis is approximately 70, at least 10,000 new cases of myelodysplastic syndrome are identified each year.
Innovation in Therapeutic Approaches Enhancing Treatment Outcomes
The introduction of hypomethylating agents, targeted therapies, and immunotherapies is reshaping the Myelodysplastic Syndrome Drugs Market. These therapies are improving survival rates, reducing disease progression, and offering patients more personalized treatment choices. Continuous research into molecular mechanisms and genetic drivers supports the advancement of new drug classes with higher efficacy. It positions pharmaceutical companies to address unmet medical needs and improve patient quality of life.
Rising Awareness and Early Diagnosis Supporting Treatment Adoption
Growing awareness among healthcare providers and patients contributes significantly to the expansion of the Myelodysplastic Syndrome Drugs Market. Early diagnosis improves treatment planning, enabling physicians to select optimal therapies at initial stages of disease progression. Campaigns from health organizations and clinical programs encourage routine blood tests and screenings, which detect abnormalities earlier. It ensures that more patients can access timely interventions that improve outcomes and reduce complications.
- For instance, Ascentage Pharma’s global Phase III GLORA-4 study for lisaftoclax in combination with azacitidine plans to enroll 464 patients with higher-risk myelodysplastic syndrome to evaluate its efficacy as a first-line treatment option.
Increasing Research and Development Investment Strengthening Market Pipeline
The Myelodysplastic Syndrome Drugs Market benefits from substantial investment in clinical trials and drug development initiatives. Pharmaceutical companies and research institutions are actively testing novel compounds that aim to enhance response rates and limit side effects. Strategic collaborations with healthcare centers and academic institutions provide platforms for innovation and faster approvals. It reinforces the market pipeline with advanced therapies designed to meet evolving patient needs.
Market Trends:
High Treatment Costs and Limited Accessibility
The Myelodysplastic Syndrome Drugs Market faces a significant challenge in the form of high treatment costs, which restrict patient access in several regions. Advanced therapies, including targeted drugs and immunotherapies, often come with premium pricing that places a heavy burden on healthcare systems and patients. It creates disparities in access, especially in low and middle-income countries where reimbursement frameworks are underdeveloped. Limited availability of specialized treatment centers further restricts adoption, leaving many patients untreated or underdiagnosed. The high economic burden also impacts long-term adherence to prescribed therapies. Addressing these cost barriers remains a critical challenge for market stakeholders seeking broader adoption.
- For instance, Novartis’ targeted therapy Tafinlar + Mekinist was studied in 57 patients with metastatic non-small cell lung cancer (NSCLC) with the BRAF V600E mutation, successfully demonstrating significant clinical effectiveness in this precise patient group.
Complexity of Disease Management and Drug Development Barriers
Managing myelodysplastic syndrome remains complex due to the heterogeneous nature of the disease, which complicates treatment selection and long-term monitoring. The Myelodysplastic Syndrome Drugs Market is impacted by challenges in developing therapies that address diverse patient needs. Clinical trials for new drugs often face high failure rates, extended timelines, and strict regulatory requirements, which delay product approvals. It also limits the number of effective therapies available in the market, slowing innovation. Physicians often struggle to balance efficacy with tolerability, especially in elderly patients with co-morbidities. These barriers highlight the need for more efficient research frameworks and collaborative efforts to overcome existing limitations.
- For instance, Agios Pharmaceuticals’ Tibsovo (ivosidenib) was granted FDA approval within about 4 years from the start of patient dosing to approval, reflecting an accelerated development timeline for a targeted therapy.
Market Challenges Analysis:
High Treatment Costs and Limited Accessibility
The Myelodysplastic Syndrome Drugs Market faces a significant challenge in the form of high treatment costs, which restrict patient access in several regions. Advanced therapies, including targeted drugs and immunotherapies, often come with premium pricing that places a heavy burden on healthcare systems and patients. It creates disparities in access, especially in low and middle-income countries where reimbursement frameworks are underdeveloped. Limited availability of specialized treatment centers further restricts adoption, leaving many patients untreated or underdiagnosed. The high economic burden also impacts long-term adherence to prescribed therapies. Addressing these cost barriers remains a critical challenge for market stakeholders seeking broader adoption.
Complexity of Disease Management and Drug Development Barriers
Managing myelodysplastic syndrome remains complex due to the heterogeneous nature of the disease, which complicates treatment selection and long-term monitoring. The Myelodysplastic Syndrome Drugs Market is impacted by challenges in developing therapies that address diverse patient needs. Clinical trials for new drugs often face high failure rates, extended timelines, and strict regulatory requirements, which delay product approvals. It also limits the number of effective therapies available in the market, slowing innovation. Physicians often struggle to balance efficacy with tolerability, especially in elderly patients with co-morbidities. These barriers highlight the need for more efficient research frameworks and collaborative efforts to overcome existing limitations.
Market Opportunities:
Expansion of Precision Medicine and Novel Therapeutics
The Myelodysplastic Syndrome Drugs Market holds strong opportunities through the growing focus on precision medicine and novel therapeutics. Advances in genomic profiling and biomarker-driven approaches allow for treatments tailored to individual patient characteristics. It creates potential for higher efficacy, fewer side effects, and improved patient adherence. Drug developers are increasingly investing in next-generation hypomethylating agents, immune checkpoint inhibitors, and combination regimens that address unmet clinical needs. Strategic collaborations with research institutes and biotech firms are further driving innovation pipelines. The rising acceptance of personalized medicine offers a clear pathway for market expansion.
Growth Potential in Emerging Healthcare Markets
Rapid improvements in healthcare infrastructure across emerging economies present significant opportunities for the Myelodysplastic Syndrome Drugs Market. Expanding access to advanced diagnostics and better insurance frameworks is improving affordability and treatment reach. It enables pharmaceutical companies to tap into large patient pools that remain underdiagnosed or untreated. Growing clinical trial activity in Asia and other developing regions also supports faster product adoption and regulatory approvals. Governments are focusing on awareness campaigns and public health programs that emphasize early detection, which boosts therapy uptake. The combination of these factors positions emerging markets as high-potential growth hubs for future expansion.
Market Segmentation Analysis:
By Treatment
The Myelodysplastic Syndrome Drugs Market is segmented by treatment, reflecting diverse therapeutic approaches tailored to patient needs. Hypomethylating agents hold a significant share due to their effectiveness in delaying disease progression and improving survival outcomes. Immunomodulatory drugs and targeted therapies are gaining traction, supported by clinical evidence and ongoing research into personalized medicine. It also benefits from the development of combination regimens that enhance treatment efficacy and address unmet clinical requirements. Supportive care drugs, including growth factors and iron chelators, remain essential in managing symptoms and improving quality of life for patients.
- For instance, Celgene’s Vidaza (azacitidine) extended median overall survival to 24.4 months compared to 15 months for conventional care in a Phase III trial.
By Route of Administration
Injectable drugs dominate the market due to their widespread use and established role in treatment regimens. Oral formulations are witnessing growth with the increasing demand for convenient, patient-friendly options that support long-term adherence. It is expected that continued innovation in drug delivery methods will enhance accessibility and expand adoption across healthcare settings. The balance between injectable and oral therapies continues to shape patient treatment pathways.
- For instance, Pfizer’s Comirnaty COVID-19 vaccine has delivered over 5 billion doses globally, demonstrating the extensive reliance on injectable therapies.
By End-Use
Hospitals lead the segment due to their ability to provide advanced therapies and specialized care. Clinics also play a growing role, particularly in delivering supportive treatments and routine monitoring. It is evident that homecare settings are gradually expanding as oral therapies and remote monitoring technologies gain acceptance. This shift offers patients greater flexibility while ensuring continuity of treatment and improved quality of care.
Segmentations:
By Treatment
- Hypomethylating Agents
- Immunomodulatory Drugs
- Targeted Therapies
- Supportive Care Drugs
By Route of Administration
By End-Use
- Hospitals
- Clinics
- Homecare Settings
By Region
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis:
Dominant Position of North America
North America accounted for 35% of the global revenue in the Myelodysplastic Syndrome Drugs Market. This leadership is supported by advanced healthcare infrastructure, high awareness among clinicians, and early adoption of innovative therapies. The region benefits from strong regulatory systems and significant research funding that accelerate the introduction of new drugs. It also hosts major pharmaceutical companies that actively expand treatment portfolios. Favorable reimbursement frameworks further improve access to costly therapies, reinforcing North America’s dominant role in the global landscape.
Strong Growth Potential in Europe
Europe represented 28% of the global revenue in the Myelodysplastic Syndrome Drugs Market. A large elderly population, structured treatment guidelines, and robust healthcare programs drive steady demand for therapies. It benefits from academic research collaborations and partnerships that advance clinical development and commercialization of new drugs. Government initiatives and patient advocacy efforts also improve awareness and early diagnosis across the region. Strategic engagement by pharmaceutical firms strengthens innovation pipelines, ensuring Europe remains a key contributor to global growth.
Rapid Expansion Across Asia Pacific
Asia Pacific captured 22% of the global revenue in the Myelodysplastic Syndrome Drugs Market and is expected to expand at the fastest pace. Rising investments in healthcare infrastructure and diagnostic technologies are improving access to therapies across the region. It benefits from government-led initiatives focused on cancer care and hematologic disease management. Increasing clinical trial activity creates opportunities for faster approvals and local innovations. The presence of a large underserved patient pool further enhances demand for advanced therapies. These factors position Asia Pacific as the most dynamic growth hub in the forecast period.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
Competitive Analysis:
The Myelodysplastic Syndrome Drugs Market is characterized by strong competition among global pharmaceutical companies, biotechnology firms, and research-driven organizations. Key players focus on advancing hypomethylating agents, targeted therapies, and immunotherapies that improve treatment efficacy and address unmet clinical needs. It reflects an industry-wide emphasis on expanding product portfolios through research investments, strategic collaborations, and licensing agreements. Many companies are strengthening clinical pipelines with next-generation drug candidates, while others are prioritizing supportive care solutions to enhance patient outcomes. Competitive positioning also relies on gaining regulatory approvals and expanding global distribution networks to capture emerging market opportunities. Growing partnerships between pharmaceutical firms and academic institutions are accelerating innovation, while mergers and acquisitions continue to shape market consolidation. This dynamic environment ensures that companies remain committed to innovation and differentiation, positioning themselves to meet rising demand and improve long-term survival outcomes for patients with myelodysplastic syndrome.
Recent Developments:
- In July 2025, Sun Pharmaceutical Industries launched LEQSELVI (deuruxolitinib) in the U.S., an anti-hair loss drug for severe alopecia areata, after resolving a patent dispute.
- In April 2025, Sun Pharma also launched FEXUCLUE® (Fexuprazan tablets 40 mg) in India for the treatment of erosive esophagitis, a novel potassium-competitive acid blocker (PCAB).
- In July 2025, Takeda announced positive Phase 3 clinical trial results for oveporexton in narcolepsy type 1, meeting all primary and secondary endpoints, demonstrating potential to transform treatment.
Report Coverage:
The research report offers an in-depth analysis based on Treatment, Route of Administration, End-Use and Region. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook:
- The Myelodysplastic Syndrome Drugs Market will see stronger adoption of targeted therapies tailored to genetic profiles.
- Hypomethylating agents are expected to maintain dominance while evolving through next-generation formulations.
- Immunotherapies will gain traction as clinical evidence supports improved survival and quality of life.
- Oral formulations will expand, offering greater convenience and supporting better patient adherence.
- Research into combination regimens will create opportunities for more effective treatment pathways.
- North America will continue to lead growth due to advanced healthcare systems and regulatory support.
- Europe will remain a key contributor, driven by structured clinical guidelines and growing elderly populations.
- Asia Pacific will emerge as a dynamic hub with rising clinical trials, patient awareness, and healthcare investment.
- Pharmaceutical collaborations with academic institutions and biotech firms will accelerate innovation pipelines.
- Rising global awareness and earlier diagnosis will expand the patient pool, supporting long-term market growth.




