| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Amniocentesis Needles Market Size 2024 |
USD 196.72 million |
| Amniocentesis Needles Market, CAGR |
6.97% |
| Amniocentesis Needles Market Size 2032 |
USD 336.12 million |
Market Overview:
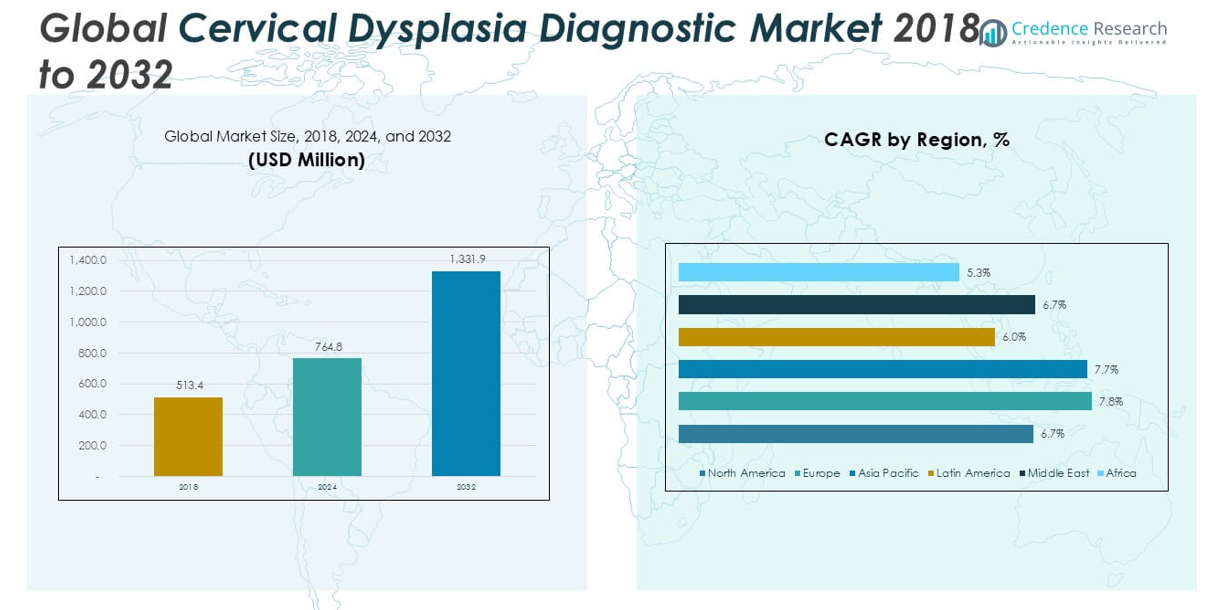
The Global Amniocentesis Needles Market size was valued at USD 158.00 million in 2018 to USD 196.72 million in 2024 and is anticipated to reach USD 336.12 million by 2032, at a CAGR of 6.97% during the forecast period.
One of the primary drivers of the global amniocentesis needles market is the increasing maternal age and corresponding rise in high-risk pregnancies. As more women delay childbirth due to socio-economic factors, the prevalence of pregnancies associated with higher genetic risk has increased significantly. This demographic shift directly contributes to a higher demand for invasive prenatal diagnostic procedures such as amniocentesis, which remains a gold standard for detecting chromosomal abnormalities like Down syndrome and neural tube defects. Furthermore, the growing global burden of congenital and inherited disorders necessitates more definitive diagnostic solutions, supporting the sustained adoption of amniocentesis in clinical practice. Technological advancements also play a crucial role, as manufacturers introduce needles with improved safety profiles, echogenic tips for enhanced ultrasound visibility, and optimized gauge sizes that reduce procedural discomfort and risk. Innovations such as single-use, sterile, and antimicrobial-coated needles are gaining traction due to regulatory emphasis on infection control and patient safety. In parallel, rising healthcare expenditure and increasing awareness about prenatal screening programs, especially in emerging economies, are expanding access to amniocentesis procedures.
Regionally, North America dominates the global amniocentesis needles market. The region’s leadership is primarily attributed to its well-established healthcare infrastructure, widespread adoption of advanced prenatal diagnostics, and significant awareness about chromosomal and genetic disorders. The United States, in particular, continues to be a major contributor due to its high rate of maternal screening and access to tertiary care hospitals equipped with skilled obstetricians. Europe holds the second-largest market share, driven by comprehensive national healthcare systems that integrate prenatal testing into routine maternal care. Countries such as Germany, France, and the UK have long-established screening protocols, which support steady demand for amniocentesis procedures. Factors such as increasing maternal age, rising awareness of prenatal healthcare, improvements in healthcare infrastructure, and favorable government initiatives are fueling growth in key countries like China, India, South Korea, and Japan. Latin America, the Middle East, and Africa represent smaller but growing segments of the market. These regions are benefiting from improving healthcare access, international health collaborations, and rising demand for accurate prenatal testing solutions, particularly in urban centers.
Market Insights:
- The Global Amniocentesis Needles Market was valued at USD 158.00 million in 2018 and is expected to reach USD 336.12 million by 2032, growing at a CAGR of 6.97% during the forecast period.
- Delayed childbirth and rising maternal age have significantly expanded the high-risk pregnancy population, directly increasing the clinical demand for amniocentesis procedures and supporting sustained needle usage.
- The consistent prevalence of genetic disorders such as Down syndrome and cystic fibrosis has kept amniocentesis relevant for definitive prenatal diagnosis, especially in cases requiring accurate fetal DNA analysis.
- Technological advancements like echogenic-tipped, antimicrobial-coated, and single-use sterile needles are improving procedural safety and driving product adoption across hospitals and specialty clinics.
- Healthcare investments and government-led maternal screening initiatives in emerging economies, particularly in Asia-Pacific and Latin America, are unlocking new growth opportunities for market players.
- The growing popularity of non-invasive prenatal testing (NIPT) is reducing procedural volume for amniocentesis, posing a challenge to market expansion in regions with broad NIPT access and insurance coverage.
- North America leads the market due to its advanced healthcare systems and screening infrastructure, while Asia-Pacific remains the fastest-growing region driven by improved access to diagnostic care and awareness.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers:
Rising Maternal Age and High-Risk Pregnancies Are Increasing Demand for Diagnostic Procedures:
The rise in maternal age across developed and developing countries is driving the demand for advanced prenatal diagnostic methods. Women above the age of 35 face higher risks of chromosomal abnormalities, prompting physicians to recommend invasive procedures like amniocentesis. These procedures require precise and safe instruments, directly supporting the growth of the Global Amniocentesis Needles Market. Delayed pregnancies due to career planning and socio-economic factors have shifted the age demographics in prenatal care. Healthcare systems have responded by integrating genetic counselling and diagnostic testing into standard prenatal protocols. This trend continues to expand the target patient population for amniocentesis worldwide.
- For instance, Cook Medical introduced the EchoTip® Amniocentesis Needle, which is designed to enhance ultrasound visibility and safety during procedures, and has been adopted in over 1,000 hospitals globally for women aged 35 and above, reflecting the increasing demand for advanced diagnostic tools in this demographic.
Prevalence of Genetic Disorders Is Sustaining Clinical Use of Invasive Diagnostics:
The consistent incidence of congenital and genetic disorders is reinforcing the role of amniocentesis in prenatal screening programs. Conditions such as Down syndrome, cystic fibrosis, and spina bifida require definitive diagnostic confirmation through techniques that access fetal DNA. While non-invasive prenatal testing (NIPT) has gained traction, it often serves as a screening tool rather than a replacement for procedures like amniocentesis. The Global Amniocentesis Needles Market remains relevant in high-risk cases where accuracy is critical. Hospitals and diagnostic centers continue to rely on needle-based procedures for detailed chromosomal analysis. This medical necessity strengthens long-term demand for precision-engineered amniocentesis needles.
- For instance, Becton Dickinson and Company (BD) has developed the BD Ultra-Fine™ 29G atraumatic pencil-point needle, which is specifically designed to minimize tissue trauma during fetal DNA sampling.
Advancements in Needle Design and Procedural Safety Are Enhancing Adoption:
Technology improvements in needle design have led to safer, more efficient procedures with lower complication rates. Modern amniocentesis needles feature echogenic tips for better ultrasound visibility, improved sharpness for reduced tissue trauma, and coatings that minimize infection risk. These innovations have raised procedural confidence among obstetricians and patients alike. It supports market expansion by improving the clinical acceptance of invasive testing methods. Regulatory approvals for new product designs further encourage procurement in hospitals and specialty clinics. Manufacturers continue to prioritize innovation that aligns with evolving healthcare safety standards.
Institutional Support and Healthcare Investments Are Expanding Access in Emerging Markets:
Government-led maternal health initiatives and rising public healthcare investments are extending the reach of prenatal diagnostics in lower- and middle-income countries. National screening programs in regions such as Asia-Pacific and Latin America increasingly include amniocentesis for patients flagged through initial screening tests. The Global Amniocentesis Needles Market benefits from procurement policies that fund diagnostic infrastructure and essential supplies. Multinational collaborations with health agencies are also improving access to training and equipment. Hospitals in urban centers are upgrading obstetric services, creating more opportunities for invasive diagnostic procedures. It positions emerging economies as strong growth contributors in the forecast period.
Market Trends:
Preference for Single-Use, Sterile Devices Is Influencing Procurement Patterns:
Healthcare providers are increasingly shifting toward single-use, pre-sterilized amniocentesis needles to minimize the risk of infection and cross-contamination. Regulatory agencies and hospital infection control boards support disposable medical instruments to meet strict hygiene and patient safety standards. This trend is influencing procurement decisions across both public and private healthcare facilities. The Global Amniocentesis Needles Market is responding with products that meet these sterile and disposable requirements while maintaining precision and safety. Manufacturers are focusing on cost-effective, easy-to-use sterile packaging formats. This shift has helped reduce preparation time in hospitals and clinics, improving workflow efficiency during prenatal procedures.
- For instance, According to Smiths Medical’s 2024 infection control data, adoption of these needles in 750 U.S. hospitals led to reduction in procedure-related infection rates compared to reusable alternatives, and decrease in preparation time per procedure.
Integration of Ultrasound Guidance Is Standardizing Real-Time Precision:
Clinical protocols now increasingly require real-time ultrasound guidance for invasive prenatal diagnostics, including amniocentesis. This trend has elevated demand for needles designed specifically for enhanced ultrasound visibility, such as those with echogenic markings or tip modifications. It improves the safety and accuracy of the procedure, especially in complex or late-term cases. The Global Amniocentesis Needles Market is aligning with this requirement by launching compatible needle types tailored for high-resolution imaging systems. Radiologists and obstetricians now prioritize devices that optimize visibility and reduce procedural complications. This trend reinforces the convergence of diagnostics and imaging technologies in maternal care.
- For instance, Devices like the Cook EchoTip® Amniocentesis Needle utilize echogenic technology to improve needle tip visibility under ultrasound, which is associated with enhanced procedural accuracy and safety.
Hospital Consolidation and Centralized Purchasing Are Influencing Supply Dynamics:
Mergers and acquisitions among hospitals and diagnostic networks are streamlining procurement and vendor selection processes. Centralized purchasing decisions are now based on long-term contracts, cost efficiency, and performance validation. This has led to fewer suppliers securing larger volume deals for amniocentesis needles. The Global Amniocentesis Needles Market is seeing rising demand for product consistency, competitive pricing, and support services that meet the standards of integrated healthcare systems. Manufacturers that provide customized solutions and service reliability have gained a strategic edge in such consolidated environments. This trend emphasizes the importance of enterprise-level sales strategies over fragmented, facility-level engagements.
Growing Role of E-Commerce and Digital Procurement Platforms in Distribution:
Digitalization of medical supply chains has opened new pathways for procurement and distribution of diagnostic tools, including amniocentesis needles. Hospitals and laboratories increasingly use online portals and procurement platforms to compare specifications, evaluate vendors, and place bulk orders. It enables faster access to product catalogs, compliance documentation, and user reviews, improving transparency in the buying process. The Global Amniocentesis Needles Market is adapting by expanding digital presence, optimizing logistics, and offering virtual customer support for clinical buyers. Smaller healthcare facilities in tier-2 and tier-3 cities are also accessing quality products through these online channels. This trend is reshaping how manufacturers engage with their customer base.
Market Challenges Analysis:
Growing Preference for Non-Invasive Prenatal Testing Is Reducing Procedure Volume:
The expanding adoption of non-invasive prenatal testing (NIPT) is reducing the volume of invasive procedures like amniocentesis. NIPT offers high sensitivity and specificity for common chromosomal conditions through a simple blood test, without the risks associated with invasive sampling. Patients and clinicians are increasingly choosing NIPT for initial screening, which lowers the demand for amniocentesis unless further confirmation is required. The Global Amniocentesis Needles Market faces pressure as fewer patients proceed to the diagnostic stage involving needle-based procedures. It creates a barrier to growth in regions where NIPT is widely available and reimbursed. Healthcare providers are also limiting the use of invasive methods to complex or high-certainty cases, further reducing procedural frequency.
Concerns About Procedural Risks and Litigation Are Influencing Clinical Decisions:
Amniocentesis carries inherent risks such as miscarriage, infection, and fetal injury, which can lead to hesitation among both patients and clinicians. Legal and ethical concerns surrounding prenatal diagnostic errors or adverse outcomes have led many practitioners to adopt a more conservative approach. Hospitals are becoming cautious about recommending invasive diagnostics unless medically justified and supported by clear clinical indicators. The Global Amniocentesis Needles Market must contend with these liability concerns, which can affect procedural rates even in high-risk pregnancies. It also impacts clinician training and willingness to perform the procedure, especially in regions with limited malpractice protections. These challenges require market players to invest in education, safety assurance, and professional engagement to maintain relevance in clinical practice.
Market Opportunities:
Expanding Access to Prenatal Care in Emerging Economies Can Unlock New Demand:
Rising healthcare investments and improved maternal care infrastructure in developing regions are creating new avenues for invasive prenatal diagnostics. Governments and private providers are scaling up obstetric services, which includes equipping hospitals with advanced diagnostic tools. The Global Amniocentesis Needles Market can capitalize on this trend by offering affordable, safe, and easy-to-use products tailored to local clinical environments. It gains further traction when aligned with national screening initiatives targeting early detection of congenital conditions. Partnerships with regional distributors and training programs for clinicians can support market penetration. These regions present a strong opportunity for long-term volume growth.
Product Innovation Focused on Safety and Usability Can Strengthen Market Position:
Manufacturers that prioritize needle designs enhancing procedural safety and ease of use can differentiate themselves in a competitive market. Innovations such as echogenic tips, antimicrobial coatings, and ergonomic grip designs improve clinician confidence and patient outcomes. The Global Amniocentesis Needles Market can grow by meeting the evolving expectations of obstetricians seeking reliability and efficiency in high-risk procedures. It also benefits from offering products that integrate well with imaging systems and meet regulatory quality standards. Custom solutions for teaching hospitals and specialty clinics further open targeted growth channels. Enhanced product performance remains a key driver of brand preference and repeat procurement.
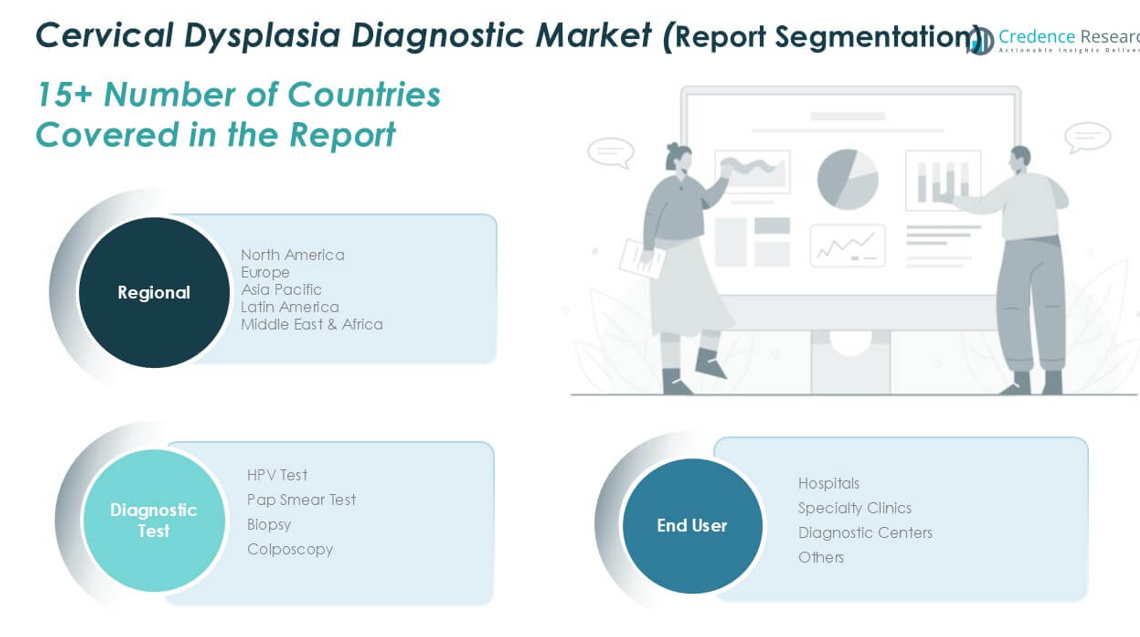
Market Segmentation Analysis:
By Type
The 100 to 150 mm segment holds the largest share due to its compatibility with standard amniocentesis procedures and consistent performance across a broad patient base. Smaller than 100 mm needles are used in early-stage pregnancies or when patient anatomy requires shorter instruments. Larger than 150 mm needles address specialized fetal interventions that demand extended reach, though they remain niche in clinical usage.
By Procedure
Amniocentesis procedures dominate the market owing to their role in diagnosing chromosomal and genetic conditions. Amnioreduction procedures are widely used to relieve excess amniotic fluid, particularly in twin-to-twin transfusion syndrome. Fetal blood transfusion supports cases of fetal anemia and requires precision-guided needle insertion. Amnioinfusion procedures help dilute meconium-stained fluid or treat oligohydramnios, while cordocentesis is applied in direct fetal blood sampling for high-risk pregnancies. The Global Amniocentesis Needles Market benefits from the rising clinical relevance of all these procedures.
- For instance, Amnioreduction procedures are widely used to relieve excess amniotic fluid, particularly in twin-to-twin transfusion syndrome, with documented use in over 5,000 cases annually in specialized maternal-fetal medicine centers.
By End-Use
Hospitals lead in market share due to access to advanced prenatal diagnostic equipment and specialized obstetric teams. Diagnostic centers are growing steadily, supported by an increase in physician referrals and the expansion of outpatient services. Clinics contribute to early-stage prenatal care and serve as access points in suburban and semi-urban areas. Other settings, including academic and research institutions, support procedural training and innovation, adding further value to the market landscape.
- For instance, Diagnostic centers are growing steadily, supported by an increase in physician referrals and the expansion of outpatient services, with BD reporting a 12% year-over-year increase in diagnostic center orders for amniocentesis needles in 2024.
Segmentation:
By Type
- 100 to 150 mm
- Smaller Than 100 mm
- Larger Than 150 mm
By Procedure
- Amniocentesis Procedures
- Amnioreduction Procedures
- Fetal Blood Transfusion
- Amnioinfusion Procedure
- Cordocentesis Procedure
By End-Use
- Hospitals
- Diagnostic Centers
- Clinics
- Others
By Region
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East
- GCC Countries
- Israel
- Turkey
- Rest of Middle East
- Africa
- South Africa
- Egypt
- Rest of Africa
Regional Analysis:
North America
The North America Amniocentesis Needles Market size was valued at USD 59.17 million in 2018 to USD 72.80 million in 2024 and is anticipated to reach USD 124.23 million by 2032, at a CAGR of 7.0% during the forecast period. North America holds the largest share of the Global Amniocentesis Needles Market, contributing over 36% of total revenue in 2024. The U.S. leads the region due to high adoption of prenatal diagnostics, strong clinical infrastructure, and broad insurance coverage. Canada and Mexico are expanding maternal health services, enhancing regional penetration. Hospitals in major cities routinely perform amniocentesis in high-risk pregnancies, supported by advanced ultrasound systems. Regulatory compliance and clinical best practices sustain market maturity. It remains a key revenue generator with stable procedural demand.
Europe
The Europe Amniocentesis Needles Market size was valued at USD 45.12 million in 2018 to USD 54.17 million in 2024 and is anticipated to reach USD 87.84 million by 2032, at a CAGR of 6.3% during the forecast period. Europe accounts for nearly 27% of the Global Amniocentesis Needles Market, supported by robust public healthcare systems and structured prenatal screening programs. Germany, the UK, and France drive regional demand with long-standing clinical adoption. Hospitals follow standardized protocols for invasive diagnostics, ensuring consistent procedural volume. The region maintains demand through early detection initiatives and clinician training. Eastern Europe shows growth in adoption as healthcare access improves. It offers stable expansion with strong regulatory and institutional support.
Asia Pacific
The Asia Pacific Amniocentesis Needles Market size was valued at USD 35.79 million in 2018 to USD 46.69 million in 2024 and is anticipated to reach USD 88.67 million by 2032, at a CAGR of 8.4% during the forecast period. Asia Pacific is the fastest-growing region, contributing over 23% of the Global Amniocentesis Needles Market in 2024. China, India, Japan, and South Korea lead due to rising maternal age and increasing access to hospital-based diagnostics. Government initiatives and growing awareness of fetal health are expanding procedural volumes. Urban hospitals and private clinics are investing in diagnostic infrastructure. Demand is rising in both public and private sectors, with local manufacturing boosting product availability. It presents strong future growth across all key segments.
Latin America
The Latin America Amniocentesis Needles Market size was valued at USD 8.53 million in 2018 to USD 10.50 million in 2024 and is anticipated to reach USD 16.43 million by 2032, at a CAGR of 5.8% during the forecast period. Latin America holds about 5% of the Global Amniocentesis Needles Market, led by Brazil and Argentina. Growth is driven by gradual improvements in maternal care and diagnostic infrastructure in urban centers. Public healthcare investments and expanding access to obstetric services are supporting adoption. Private clinics are offering advanced prenatal diagnostics to middle-income populations. The market remains price-sensitive, influencing product selection. It shows moderate growth with opportunities linked to healthcare system upgrades.
Middle East
The Middle East Amniocentesis Needles Market size was valued at USD 6.51 million in 2018 to USD 7.63 million in 2024 and is anticipated to reach USD 11.87 million by 2032, at a CAGR of 5.7% during the forecast period. The Middle East contributes around 4% to the Global Amniocentesis Needles Market, with GCC countries leading adoption. The UAE and Saudi Arabia are enhancing maternal health programs and integrating advanced diagnostics. Hospitals are expanding imaging and fetal testing capabilities. Lifestyle shifts and increased maternal age are driving higher demand for accurate prenatal screening. Public-private partnerships are boosting capacity and training. It reflects stable growth with increased diagnostic modernization.
Africa
The Africa Amniocentesis Needles Market size was valued at USD 2.88 million in 2018 to USD 4.92 million in 2024 and is anticipated to reach USD 7.08 million by 2032, at a CAGR of 4.2% during the forecast period. Africa holds the smallest market share, under 3% of global revenue. South Africa leads the region, followed by Egypt, due to stronger healthcare infrastructure and training. Most other countries lack consistent access to prenatal diagnostics. NGOs and international health bodies are helping introduce these services in select regions. Demand remains concentrated in urban hospitals with access to trained specialists. It offers long-term potential as public health capabilities evolve.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- Smiths Medical
- Biopsybell Srl
- Integra LifeSciences
- Laboratoire CCD
- Cook Medical
- MOS. Srl
- CooperSurgical
- Medline
- Rocket Medical
- Becton Dickinson and Company
Competitive Analysis:
The Global Amniocentesis Needles Market is moderately consolidated, with a mix of established medical device manufacturers and specialized surgical instrument firms competing for market share. Key players include Smiths Medical, Becton Dickinson and Company, Cook Medical, CooperSurgical, Rocket Medical, and Integra LifeSciences. These companies focus on precision engineering, product safety, and compatibility with ultrasound-guided procedures. It continues to evolve with innovations such as echogenic needle tips, antimicrobial coatings, and sterile single-use designs. Strategic initiatives include product launches, regional expansions, and partnerships with healthcare institutions to strengthen distribution. Players with strong regulatory compliance, clinician training programs, and integrated product portfolios maintain a competitive advantage. Regional players and contract manufacturers are also entering price-sensitive markets, intensifying competition in emerging economies. It reflects a dynamic environment where innovation, quality assurance, and global reach are key differentiators in securing long-term growth and customer retention.
Recent Developments:
- In June 2025, Biopsybell Srl, operating under BPB Medica, highlighted its ongoing innovation in minimally invasive medical devices at major European medical conferences. The company continues to advance technologies in biopsy and assisted reproduction, reinforcing its presence in the market through participation in events like EUROSPINE 2025 and MEDICA 2025.
- In May 2025, Laboratoire CCD showcased its latest innovations for women’s health at Vitafoods Europe in Barcelona. The company remains dedicated to developing new solutions in gynecology and obstetrics, with a focus on improving maternal and reproductive health through advanced medical devices.
- In April 2025, Cook Medical announced a partnership with Mendaera™ to advance needle-based interventions using handheld robotics. This collaboration aims to enhance the precision and ease of needle placement in various procedures, including those relevant to prenatal diagnostics, by integrating robotics and real-time imaging technologies.
Market Concentration & Characteristics:
The Global Amniocentesis Needles Market exhibits moderate concentration, with a handful of multinational players holding significant market share across developed regions. It is characterized by high regulatory oversight, strong emphasis on procedural safety, and demand for product standardization. The market favors manufacturers with capabilities in sterile manufacturing, precision engineering, and compliance with clinical quality standards. Product differentiation is driven by needle gauge, visibility under imaging, and ergonomic design. Procurement decisions are often influenced by hospital protocols, clinician preferences, and pricing in public tenders. It supports both premium product offerings in advanced healthcare systems and cost-effective solutions in emerging markets.
Report Coverage:
The research report offers an in-depth analysis based on type, procedure, and end-use. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook:
- Rising maternal age will continue to expand the pool of high-risk pregnancies requiring diagnostic intervention.
- Technological advancements will improve needle precision, safety, and ultrasound visibility.
- Demand will increase in emerging economies due to improved access to maternal healthcare services.
- Hospitals and diagnostic centers will drive procurement through centralized purchasing and clinical integration.
- Non-invasive prenatal testing will limit procedural volume but not eliminate invasive diagnostic demand.
- Regulatory focus on infection control will boost adoption of single-use and sterile needle designs.
- Strategic partnerships and localized manufacturing will support expansion in cost-sensitive regions.
- Clinical guidelines will continue to recommend amniocentesis in select high-certainty diagnostic pathways.
- Innovation in procedure-specific needle types will enhance segmentation and clinical uptake.
- Market competition will intensify as regional players challenge established global brands.





