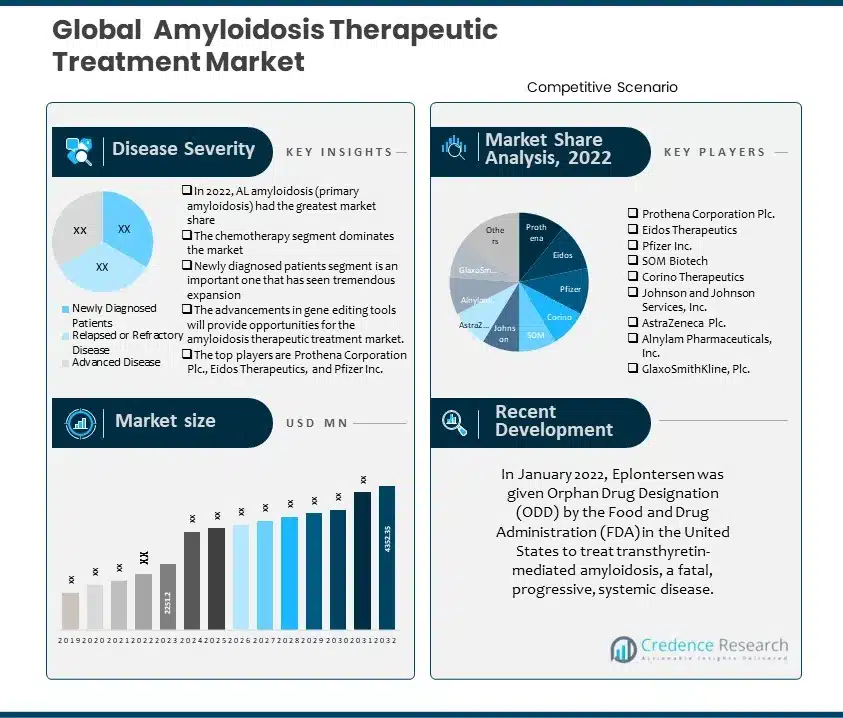
1. Preface
1.1. Report Description
1.1.1. Purpose of the Report
1.1.2. Target Audience
1.1.3. USP and Key Offerings
1.2. Research Scope
1.3. Market Introduction
2. Executive Summary
2.1. Market Snapshot: Global Amyloidosis Therapeutic Treatment Market
2.1.1. Global Amyloidosis Therapeutic Treatment Market, By Type of Amyloidosis
2.1.2. Global Amyloidosis Therapeutic Treatment Market, By Treatment Modalities
2.1.3. Global Amyloidosis Therapeutic Treatment Market, By Disease Severity
2.1.4. Global Amyloidosis Therapeutic Treatment Market, By Region
2.2. Insights from Primary Respondents
3. Market Dynamics & Factors Analysis
3.1. Introduction
3.1.1. Global Amyloidosis Therapeutic Treatment Market Value, 2019-2032, (US$ Mn)
3.1.2. Y-o-Y Growth Trend Analysis
3.2. Market Dynamics
3.2.1. Amyloidosis Therapeutic Treatment Market Drivers
3.2.2. Amyloidosis Therapeutic Treatment Market Restraints
3.2.3. Amyloidosis Therapeutic Treatment Market Opportunities
3.2.4. Major Amyloidosis Therapeutic Treatment Industry Challenges
3.3. Growth and Development Patterns
3.4. Investment Feasibility Analysis
3.5. Market Opportunity Analysis
3.5.1. Type of Amyloidosis
3.5.2. Treatment Modalities
3.5.3. Disease Severity
3.5.4. Geography
4. Market Competitive Landscape Analysis
4.1. Company Market Share Analysis, 2023
4.1.1. Global Amyloidosis Therapeutic Treatment Market: Company Market Share, Value 2023
4.1.2. Global Amyloidosis Therapeutic Treatment Market: Top 6 Company Market Share, Value 2023
4.1.3. Global Amyloidosis Therapeutic Treatment Market: Top 3 Company Market Share, Value 2023
4.2. Global Amyloidosis Therapeutic Treatment Market: Company Revenue Share Analysis, 2023
4.3. Company Assessment Metrics, 2023
4.3.1. Stars
4.3.2. Emerging Leaders
4.3.3. Pervasive Players
4.3.4. Participants
4.4. Startups/ SMEs Assessment Metrics, 2023
4.4.1. Progressive Companies
4.4.2. Responsive Companies
4.4.3. Dynamic Companies
4.4.4. Starting Blocks
4.5. Strategic Development
4.5.1. Acquisition and Mergers
4.5.2. New Product Launch
4.5.3. Regional Expansion
4.5.4. Partnerships
4.6. Key Player Product Matrix
4.7. Potential for New Players in the Global Amyloidosis Therapeutic Treatment Market
5. Premium Insights
5.1. STAR (Situation, Task, Action, Results) Analysis
5.2. Porter’s Five Forces Analysis
5.2.1. Threat of New Entrants
5.2.2. Bargaining Power of Buyers/Consumers
5.2.3. Bargaining Power of Suppliers
5.2.4. Threat of Substitute Types
5.2.5. Intensity of Competitive Rivalry
5.3. PESTEL Analysis
5.3.1. Political Factors
5.3.2. Economic Factors
5.3.3. Social Factors
5.3.4. Technological Factors
5.3.5. Environmental Factors
5.3.6. Legal Factors
5.4. Key Market Trends
5.4.1. Demand Side Trends
5.4.2. Supply Side Trends
5.5. Value Chain Analysis
5.6. Technology Analysis
5.6.1. Research and development in the global market
5.6.2. Patent Analysis
5.6.3. Emerging technologies and their potential disruption to the market
5.7. Consumer Behaviour Analysis
5.7.1. Consumer Preferences and Expectations
5.7.2. Factors Influencing Consumer Buying Decisions
5.7.2.1. North America
5.7.2.2. Europe
5.7.2.3. Asia Pacific
5.7.2.4. Latin America
5.7.2.5. Middle East and Africa
5.7.3. Consumer Pain Points
5.8. Analysis and Recommendations
5.9. Adjacent Market Analysis
6. Market Positioning of Key Players, 2023
6.1. Company market share of key players, 2023
6.2. Competitive Benchmarking
6.3. Market Positioning of Key Vendors
6.4. Geographical Presence Analysis
6.5. Major Strategies Adopted by Key Players
6.5.1. Key Strategies Analysis
6.5.2. Mergers and Acquisitions
6.5.3. Partnerships
6.5.4. Product Launch
6.5.5. Geographical Expansion
6.5.6. Others
7. Impact Analysis of COVID 19 and Russia – Ukraine War on Amyloidosis Therapeutic Treatment Market
7.1. Ukraine-Russia War Impact
7.1.1. Uncertainty and Economic Instability
7.1.2. Supply chain disruptions
7.1.3. Regional market shifts
7.1.4. Shift in government priorities
7.2. COVID-19 Impact Analysis
7.2.1. Supply Chain Disruptions
7.2.2. Demand Fluctuations
7.2.3. Shift in Product Mix
7.2.4. Reduced Industrial Activity
7.2.5. Regional Impact Analysis
7.2.5.1. North America
7.2.5.2. Europe
7.2.5.3. Asia Pacific
7.2.5.4. Latin America
7.2.5.5. Middle East and Africa
8. Global Amyloidosis Therapeutic Treatment Market, By Type of Amyloidosis
8.1. Global Amyloidosis Therapeutic Treatment Market Overview, by Type of Amyloidosis
8.1.1. Global Amyloidosis Therapeutic Treatment Market Revenue Share, By Type of Amyloidosis, 2023 Vs 2032 (in %)
8.2. AL Amyloidosis (Primary Amyloidosis)
8.2.1. Global Amyloidosis Therapeutic Treatment Market, By AL Amyloidosis (Primary Amyloidosis), By Region, 2019-2032 (US$ Mn)
8.2.2. Market Dynamics for AL Amyloidosis (Primary Amyloidosis)
8.2.2.1. Drivers
8.2.2.2. Restraints
8.2.2.3. Opportunities
8.2.2.4. Trends
8.3. ATTR Amyloidosis (Hereditary and Wild-Type)
8.3.1. Global Amyloidosis Therapeutic Treatment Market, By ATTR Amyloidosis (Hereditary and Wild-Type), By Region, 2019-2032 (US$ Mn)
8.3.2. Market Dynamics for ATTR Amyloidosis (Hereditary and Wild-Type)
8.3.2.1. Drivers
8.3.2.2. Restraints
8.3.2.3. Opportunities
8.3.2.4. Trends
8.4. AA Amyloidosis (Secondary Amyloidosis)
8.4.1. Global Amyloidosis Therapeutic Treatment Market, By AA Amyloidosis (Secondary Amyloidosis), By Region, 2019-2032 (US$ Mn)
8.4.2. Market Dynamics for AA Amyloidosis (Secondary Amyloidosis)
8.4.2.1. Drivers
8.4.2.2. Restraints
8.4.2.3. Opportunities
8.4.2.4. Trends
9. Global Amyloidosis Therapeutic Treatment Market, By Treatment Modalities
9.1. Global Amyloidosis Therapeutic Treatment Market Overview, by Treatment Modalities
9.1.1. Global Amyloidosis Therapeutic Treatment Market Revenue Share, By Treatment Modalities, 2023 Vs 2032 (in %)
9.2. Chemotherapy
9.2.1. Global Amyloidosis Therapeutic Treatment Market, By Chemotherapy, By Region, 2019-2032 (US$ Mn)
9.2.2. Market Dynamics for Chemotherapy
9.2.2.1. Drivers
9.2.2.2. Restraints
9.2.2.3. Opportunities
9.2.2.4. Trends
9.3. Immunomodulatory Drugs (IMiDs)
9.3.1. Global Amyloidosis Therapeutic Treatment Market, By Immunomodulatory Drugs (IMiDs), By Region, 2019-2032 (US$ Mn)
9.3.2. Market Dynamics for Immunomodulatory Drugs (IMiDs)
9.3.2.1. Drivers
9.3.2.2. Restraints
9.3.2.3. Opportunities
9.3.2.4. Trends
9.4. Monoclonal Antibodies
9.4.1. Global Amyloidosis Therapeutic Treatment Market, By Monoclonal Antibodies, By Region, 2019-2032 (US$ Mn)
9.4.2. Market Dynamics for Monoclonal Antibodies
9.4.2.1. Drivers
9.4.2.2. Restraints
9.4.2.3. Opportunities
9.4.2.4. Trends
9.5. TTR Stabilizers
9.5.1. Global Amyloidosis Therapeutic Treatment Market, By TTR Stabilizers, By Region, 2019-2032 (US$ Mn)
9.5.2. Market Dynamics for TTR Stabilizers
9.5.2.1. Drivers
9.5.2.2. Restraints
9.5.2.3. Opportunities
9.5.2.4. Trends
9.6. RNA Interference (RNAi) Therapies
9.6.1. Global Amyloidosis Therapeutic Treatment Market, By RNA Interference (RNAi) Therapies, By Region, 2019-2032 (US$ Mn)
9.6.2. Market Dynamics for RNA Interference (RNAi) Therapies
9.6.2.1. Drivers
9.6.2.2. Restraints
9.6.2.3. Opportunities
9.6.2.4. Trends
9.7. Liver Transplantation
9.7.1. Global Amyloidosis Therapeutic Treatment Market, By Liver Transplantation, By Region, 2019-2032 (US$ Mn)
9.7.2. Market Dynamics for Liver Transplantation
9.7.2.1. Drivers
9.7.2.2. Restraints
9.7.2.3. Opportunities
9.7.2.4. Trends
9.8. Supportive Care
9.8.1. Global Amyloidosis Therapeutic Treatment Market, By Supportive Care, By Region, 2019-2032 (US$ Mn)
9.8.2. Market Dynamics for Supportive Care
9.8.2.1. Drivers
9.8.2.2. Restraints
9.8.2.3. Opportunities
9.8.2.4. Trends
10. Global Amyloidosis Therapeutic Treatment Market, By Disease Severity
10.1. Global Amyloidosis Therapeutic Treatment Market Overview, by Disease Severity
10.1.1. Global Amyloidosis Therapeutic Treatment Market Revenue Share, By Disease Severity, 2023 Vs 2032 (in %)
10.2. Newly Diagnosed Patients
10.2.1. Global Amyloidosis Therapeutic Treatment Market, By Newly Diagnosed Patients, By Region, 2019-2032 (US$ Mn)
10.2.2. Market Dynamics for Newly Diagnosed Patients
10.2.2.1. Drivers
10.2.2.2. Restraints
10.2.2.3. Opportunities
10.2.2.4. Trends
10.3. Relapsed or Refractory Disease
10.3.1. Global Amyloidosis Therapeutic Treatment Market, By Relapsed or Refractory Disease, By Region, 2019-2032 (US$ Mn)
10.3.2. Market Dynamics for Relapsed or Refractory Disease
10.3.2.1. Drivers
10.3.2.2. Restraints
10.3.2.3. Opportunities
10.3.2.4. Trends
10.4. Advanced Disease
10.4.1. Global Amyloidosis Therapeutic Treatment Market, By Advanced Disease, By Region, 2019-2032 (US$ Mn)
10.4.2. Market Dynamics for Advanced Disease
10.4.2.1. Drivers
10.4.2.2. Restraints
10.4.2.3. Opportunities
10.4.2.4. Trends
11. Global Amyloidosis Therapeutic Treatment Market, By Region
11.1. Global Amyloidosis Therapeutic Treatment Market Overview, by Region
11.1.1. Global Amyloidosis Therapeutic Treatment Market, By Region, 2023 Vs 2032 (in%)
11.2. Type of Amyloidosis
11.2.1. Global Amyloidosis Therapeutic Treatment Market, By Type of Amyloidosis, 2019-2032 (US$ Mn)
11.3. Treatment Modalities
11.3.1. Global Amyloidosis Therapeutic Treatment Market, By Treatment Modalities, 2019-2032 (US$ Mn)
11.4. Disease Severity
11.4.1. Global Amyloidosis Therapeutic Treatment Market, By Disease Severity, 2019-2032 (US$ Mn)
12. North America Amyloidosis Therapeutic Treatment Market Analysis
12.1. Overview
12.1.1. Market Dynamics for North America
12.1.1.1. Drivers
12.1.1.2. Restraints
12.1.1.3. Opportunities
12.1.1.4. Trends
12.2. North America Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2032(US$ Mn)
12.2.1. Overview
12.2.2. SRC Analysis
12.3. North America Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2032(US$ Mn)
12.3.1. Overview
12.3.2. SRC Analysis
12.4. North America Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2032(US$ Mn)
12.4.1. Overview
12.4.2. SRC Analysis
12.5. North America Amyloidosis Therapeutic Treatment Market, by Country, 2019-2032 (US$ Mn)
12.5.1. North America Amyloidosis Therapeutic Treatment Market, by Country, 2023 Vs 2032 (in%)
12.5.2. U.S.
12.5.3. Canada
12.5.4. Mexico
13. Europe Amyloidosis Therapeutic Treatment Market Analysis
13.1. Overview
13.1.1. Market Dynamics for Europe
13.1.1.1. Drivers
13.1.1.2. Restraints
13.1.1.3. Opportunities
13.1.1.4. Trends
13.2. Europe Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2032(US$ Mn)
13.2.1. Overview
13.2.2. SRC Analysis
13.3. Europe Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2032(US$ Mn)
13.3.1. Overview
13.3.2. SRC Analysis
13.4. Europe Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2032(US$ Mn)
13.4.1. Overview
13.4.2. SRC Analysis
13.5. Europe Amyloidosis Therapeutic Treatment Market, by Country, 2019-2032 (US$ Mn)
13.5.1. Europe Amyloidosis Therapeutic Treatment Market, by Country, 2023 Vs 2032 (in%)
13.5.2. UK
13.5.3. France
13.5.4. Germany
13.5.5. Italy
13.5.6. Spain
13.5.7. Benelux
13.5.8. Russia
13.5.9. Rest of Europe
14. Asia Pacific Amyloidosis Therapeutic Treatment Market Analysis
14.1. Overview
14.1.1. Market Dynamics for Asia Pacific
14.1.1.1. Drivers
14.1.1.2. Restraints
14.1.1.3. Opportunities
14.1.1.4. Trends
14.2. Asia Pacific Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2032(US$ Mn)
14.2.1. Overview
14.2.2. SRC Analysis
14.3. Asia Pacific Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2032(US$ Mn)
14.3.1. Overview
14.3.2. SRC Analysis
14.4. Asia Pacific Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2032(US$ Mn)
14.4.1. Overview
14.4.2. SRC Analysis
14.5. Asia Pacific Amyloidosis Therapeutic Treatment Market, by Country, 2019-2032 (US$ Mn)
14.5.1. Asia Pacific Amyloidosis Therapeutic Treatment Market, by Country, 2023 Vs 2032 (in%)
14.5.2. China
14.5.3. Japan
14.5.4. India
14.5.5. South Korea
14.5.6. South East Asia
14.5.7. Rest of Asia Pacific
15. Latin America Amyloidosis Therapeutic Treatment Market Analysis
15.1. Overview
15.1.1. Market Dynamics for Latin America
15.1.1.1. Drivers
15.1.1.2. Restraints
15.1.1.3. Opportunities
15.1.1.4. Trends
15.2. Latin America Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2032(US$ Mn)
15.2.1. Overview
15.2.2. SRC Analysis
15.3. Latin America Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2032(US$ Mn)
15.3.1. Overview
15.3.2. SRC Analysis
15.4. Latin America Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2032(US$ Mn)
15.4.1. Overview
15.4.2. SRC Analysis
15.5. Latin America Amyloidosis Therapeutic Treatment Market, by Country, 2019-2032 (US$ Mn)
15.5.1. Latin America Amyloidosis Therapeutic Treatment Market, by Country, 2023 Vs 2032 (in%)
15.5.2. Brazil
15.5.3. Argentina
15.5.4. Rest of Latin America
16. Middle East Amyloidosis Therapeutic Treatment Market Analysis
16.1. Overview
16.1.1. Market Dynamics for Middle East
16.1.1.1. Drivers
16.1.1.2. Restraints
16.1.1.3. Opportunities
16.1.1.4. Trends
16.2. Middle East Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2032(US$ Mn)
16.2.1. Overview
16.2.2. SRC Analysis
16.3. Middle East Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2032(US$ Mn)
16.3.1. Overview
16.3.2. SRC Analysis
16.4. Middle East Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2032(US$ Mn)
16.4.1. Overview
16.4.2. SRC Analysis
16.5. Middle East Amyloidosis Therapeutic Treatment Market, by Country, 2019-2032 (US$ Mn)
16.5.1. Middle East Amyloidosis Therapeutic Treatment Market, by Country, 2023 Vs 2032 (in%)
16.5.2. UAE
16.5.3. Saudi Arabia
16.5.4. Rest of Middle East
17. Africa Amyloidosis Therapeutic Treatment Market Analysis
17.1. Overview
17.1.1. Market Dynamics for Africa
17.1.1.1. Drivers
17.1.1.2. Restraints
17.1.1.3. Opportunities
17.1.1.4. Trends
17.2. Africa Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2032(US$ Mn)
17.2.1. Overview
17.2.2. SRC Analysis
17.3. Africa Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2032(US$ Mn)
17.3.1. Overview
17.3.2. SRC Analysis
17.4. Africa Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2032(US$ Mn)
17.4.1. Overview
17.4.2. SRC Analysis
17.5. Africa Amyloidosis Therapeutic Treatment Market, by Country, 2019-2032 (US$ Mn)
17.5.1. Africa Amyloidosis Therapeutic Treatment Market, by Country, 2023 Vs 2032 (in%)
17.5.2. South Africa
17.5.3. Egypt
17.5.4. Rest of Africa
18. Company Profiles
18.1. Prothena Corporation Plc.
18.1.1. Company Overview
18.1.2. Products/Services Portfolio
18.1.3. Geographical Presence
18.1.4. SWOT Analysis
18.1.5. Financial Summary
18.1.5.1. Market Revenue and Net Profit (2019-2023)
18.1.5.2. Business Segment Revenue Analysis
18.1.5.3. Geographical Revenue Analysis
18.2. Eidos Therapeutics
18.3. Pfizer Inc.
18.4. SOM Biotech
18.5. Corino Therapeutics
18.6. Johnson and Johnson Services, Inc.
18.7. AstraZeneca Plc.
18.8. Alnylam Pharmaceuticals, Inc.
18.9. GlaxoSmithKline, Plc.
19. Research Methodology
19.1. Research Methodology
19.2. Phase I – Secondary Research
19.3. Phase II – Data Modelling
19.3.1. Company Share Analysis Model
19.3.2. Revenue Based Modelling
19.4. Phase III – Primary Research
19.5. Research Limitations
19.5.1. Assumptions
List of Figures
FIG. 1 Global Amyloidosis Therapeutic Treatment Market: Research Methodology
FIG. 2 Market Size Estimation – Top Down & Bottom up Approach
FIG. 3 Global Amyloidosis Therapeutic Treatment Market Segmentation
FIG. 4 Global Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2023 (US$ Mn)
FIG. 5 Global Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2023 (US$ Mn)
FIG. 6 Global Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2023 (US$ Mn)
FIG. 7 Global Amyloidosis Therapeutic Treatment Market, by Geography, 2023 (US$ Mn)
FIG. 8 Attractive Investment Proposition, by Type of Amyloidosis, 2023
FIG. 9 Attractive Investment Proposition, by Treatment Modalities, 2023
FIG. 10 Attractive Investment Proposition, by Disease Severity, 2023
FIG. 11 Attractive Investment Proposition, by Geography, 2023
FIG. 12 Global Market Share Analysis of Key Amyloidosis Therapeutic Treatment Market Manufacturers, 2023
FIG. 13 Global Market Positioning of Key Amyloidosis Therapeutic Treatment Market Manufacturers, 2023
FIG. 14 Global Amyloidosis Therapeutic Treatment Market Value Contribution, By Type of Amyloidosis, 2023 & 2032 (Value %)
FIG. 15 Global Amyloidosis Therapeutic Treatment Market, by AL Amyloidosis (Primary Amyloidosis), Value, 2019-2032 (US$ Mn)
FIG. 16 Global Amyloidosis Therapeutic Treatment Market, by ATTR Amyloidosis (Hereditary and Wild-Type), Value, 2019-2032 (US$ Mn)
FIG. 17 Global Amyloidosis Therapeutic Treatment Market, by AA Amyloidosis (Secondary Amyloidosis), Value, 2019-2032 (US$ Mn)
FIG. 18 Global Amyloidosis Therapeutic Treatment Market Value Contribution, By Treatment Modalities, 2023 & 2032 (Value %)
FIG. 19 Global Amyloidosis Therapeutic Treatment Market, by Chemotherapy, Value, 2019-2032 (US$ Mn)
FIG. 20 Global Amyloidosis Therapeutic Treatment Market, by Immunomodulatory Drugs (IMiDs), Value, 2019-2032 (US$ Mn)
FIG. 21 Global Amyloidosis Therapeutic Treatment Market, by Monoclonal Antibodies, Value, 2019-2032 (US$ Mn)
FIG. 22 Global Amyloidosis Therapeutic Treatment Market, by TTR Stabilizers, Value, 2019-2032 (US$ Mn)
FIG. 23 Global Amyloidosis Therapeutic Treatment Market, by RNA Interference (RNAi) Therapies, Value, 2019-2032 (US$ Mn)
FIG. 24 Global Amyloidosis Therapeutic Treatment Market, by Liver Transplantation, Value, 2019-2032 (US$ Mn)
FIG. 25 Global Amyloidosis Therapeutic Treatment Market, by Supportive Care, Value, 2019-2032 (US$ Mn)
FIG. 26 Global Amyloidosis Therapeutic Treatment Market Value Contribution, By Disease Severity, 2023 & 2032 (Value %)
FIG. 27 Global Amyloidosis Therapeutic Treatment Market, by Newly Diagnosed Patients, Value, 2019-2032 (US$ Mn)
FIG. 28 Global Amyloidosis Therapeutic Treatment Market, by Relapsed or Refractory Disease, Value, 2019-2032 (US$ Mn)
FIG. 29 Global Amyloidosis Therapeutic Treatment Market, by Advanced Disease, Value, 2019-2032 (US$ Mn)
FIG. 30 North America Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 31 U.S. Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 32 Canada Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 33 Mexico Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 34 Europe Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 35 Germany Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 36 France Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 37 U.K. Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 38 Italy Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 39 Spain Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 40 Benelux Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 41 Russia Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 42 Rest of Europe Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 43 Asia Pacific Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 44 China Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 45 Japan Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 46 India Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 47 South Korea Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 48 South-East Asia Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 49 Rest of Asia Pacific Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 50 Latin America Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 51 Brazil Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 52 Argentina Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 53 Rest of Latin America Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 54 Middle East Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 55 UAE Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 56 Saudi Arabia Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 57 Rest of Middle East Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 58 Africa Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 59 South Africa Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 60 Egypt Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
FIG. 61 Rest of Africa Amyloidosis Therapeutic Treatment Market, 2019-2032 (US$ Mn)
List of Tables
TABLE 1 Market Snapshot: Global Amyloidosis Therapeutic Treatment Market
TABLE 2 Global Amyloidosis Therapeutic Treatment Market: Market Drivers Impact Analysis
TABLE 3 Global Amyloidosis Therapeutic Treatment Market: Market Restraints Impact Analysis
TABLE 4 Global Amyloidosis Therapeutic Treatment Market, by Competitive Benchmarking, 2023
TABLE 5 Global Amyloidosis Therapeutic Treatment Market, by Geographical Presence Analysis, 2023
TABLE 6 Global Amyloidosis Therapeutic Treatment Market, by Key Strategies Analysis, 2023
TABLE 7 Global Amyloidosis Therapeutic Treatment Market, by AL Amyloidosis (Primary Amyloidosis), By Region, 2019-2023 (US$ Mn)
TABLE 8 Global Amyloidosis Therapeutic Treatment Market, by AL Amyloidosis (Primary Amyloidosis), By Region, 2024-2032 (US$ Mn)
TABLE 9 Global Amyloidosis Therapeutic Treatment Market, by ATTR Amyloidosis (Hereditary and Wild-Type), By Region, 2019-2023 (US$ Mn)
TABLE 10 Global Amyloidosis Therapeutic Treatment Market, by ATTR Amyloidosis (Hereditary and Wild-Type), By Region, 2024-2032 (US$ Mn)
TABLE 11 Global Amyloidosis Therapeutic Treatment Market, by AA Amyloidosis (Secondary Amyloidosis), By Region, 2019-2023 (US$ Mn)
TABLE 12 Global Amyloidosis Therapeutic Treatment Market, by AA Amyloidosis (Secondary Amyloidosis), By Region, 2024-2032 (US$ Mn)
TABLE 13 Global Amyloidosis Therapeutic Treatment Market, by Chemotherapy, By Region, 2019-2023 (US$ Mn)
TABLE 14 Global Amyloidosis Therapeutic Treatment Market, by Chemotherapy, By Region, 2024-2032 (US$ Mn)
TABLE 15 Global Amyloidosis Therapeutic Treatment Market, by Immunomodulatory Drugs (IMiDs), By Region, 2019-2023 (US$ Mn)
TABLE 16 Global Amyloidosis Therapeutic Treatment Market, by Immunomodulatory Drugs (IMiDs), By Region, 2024-2032 (US$ Mn)
TABLE 17 Global Amyloidosis Therapeutic Treatment Market, by Monoclonal Antibodies, By Region, 2019-2023 (US$ Mn)
TABLE 18 Global Amyloidosis Therapeutic Treatment Market, by Monoclonal Antibodies, By Region, 2024-2032 (US$ Mn)
TABLE 19 Global Amyloidosis Therapeutic Treatment Market, by TTR Stabilizers, By Region, 2019-2023 (US$ Mn)
TABLE 20 Global Amyloidosis Therapeutic Treatment Market, by TTR Stabilizers, By Region, 2024-2032 (US$ Mn)
TABLE 21 Global Amyloidosis Therapeutic Treatment Market, by RNA Interference (RNAi) Therapies, By Region, 2019-2023 (US$ Mn)
TABLE 22 Global Amyloidosis Therapeutic Treatment Market, by RNA Interference (RNAi) Therapies, By Region, 2024-2032 (US$ Mn)
TABLE 23 Global Amyloidosis Therapeutic Treatment Market, by Liver Transplantation, By Region, 2019-2023 (US$ Mn)
TABLE 24 Global Amyloidosis Therapeutic Treatment Market, by Liver Transplantation, By Region, 2024-2032 (US$ Mn)
TABLE 25 Global Amyloidosis Therapeutic Treatment Market, by Supportive Care, By Region, 2019-2023 (US$ Mn)
TABLE 26 Global Amyloidosis Therapeutic Treatment Market, by Supportive Care, By Region, 2024-2032 (US$ Mn)
TABLE 27 Global Amyloidosis Therapeutic Treatment Market, by Newly Diagnosed Patients, By Region, 2019-2023 (US$ Mn)
TABLE 28 Global Amyloidosis Therapeutic Treatment Market, by Newly Diagnosed Patients, By Region, 2024-2032 (US$ Mn)
TABLE 29 Global Amyloidosis Therapeutic Treatment Market, by Relapsed or Refractory Disease, By Region, 2019-2023 (US$ Mn)
TABLE 30 Global Amyloidosis Therapeutic Treatment Market, by Relapsed or Refractory Disease, By Region, 2024-2032 (US$ Mn)
TABLE 31 Global Amyloidosis Therapeutic Treatment Market, by Advanced Disease, By Region, 2019-2023 (US$ Mn)
TABLE 32 Global Amyloidosis Therapeutic Treatment Market, by Advanced Disease, By Region, 2024-2032 (US$ Mn)
TABLE 33 Global Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 34 Global Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 35 Global Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 36 Global Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 37 Global Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 38 Global Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 39 Global Amyloidosis Therapeutic Treatment Market, by Region, 2019-2023 (US$ Mn)
TABLE 40 Global Amyloidosis Therapeutic Treatment Market, by Region, 2024-2032 (US$ Mn)
TABLE 41 North America Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 42 North America Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 43 North America Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 44 North America Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 45 North America Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 46 North America Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 47 North America Amyloidosis Therapeutic Treatment Market, by Country, 2019-2023 (US$ Mn)
TABLE 48 North America Amyloidosis Therapeutic Treatment Market, by Country, 2024-2032 (US$ Mn)
TABLE 49 United States Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 50 United States Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 51 United States Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 52 United States Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 53 United States Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 54 United States Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 55 Canada Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 56 Canada Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 57 Canada Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 58 Canada Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 59 Canada Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 60 Canada Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 61 Mexico Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 62 Mexico Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 63 Mexico Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 64 Mexico Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 65 Mexico Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 66 Mexico Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 67 Europe Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 68 Europe Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 69 Europe Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 70 Europe Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 71 Europe Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 72 Europe Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 73 Europe Amyloidosis Therapeutic Treatment Market, by Country, 2019-2023 (US$ Mn)
TABLE 74 Europe Amyloidosis Therapeutic Treatment Market, by Country, 2024-2032 (US$ Mn)
TABLE 75 Germany Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 76 Germany Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 77 Germany Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 78 Germany Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 79 Germany Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 80 Germany Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 81 France Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 82 France Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 83 France Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 84 France Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 85 France Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 86 France Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 87 United Kingdom Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 88 United Kingdom Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 89 United Kingdom Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 90 United Kingdom Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 91 United Kingdom Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 92 United Kingdom Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 93 Italy Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 94 Italy Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 95 Italy Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 96 Italy Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 97 Italy Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 98 Italy Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 99 Spain Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 100 Spain Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 101 Spain Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 102 Spain Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 103 Spain Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 104 Spain Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 105 Benelux Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 106 Benelux Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 107 Benelux Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 108 Benelux Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 109 Benelux Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 110 Benelux Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 111 Russia Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 112 Russia Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 113 Russia Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 114 Russia Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 115 Russia Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 116 Russia Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 117 Rest of Europe Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 118 Rest of Europe Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 119 Rest of Europe Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 120 Rest of Europe Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 121 Rest of Europe Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 122 Rest of Europe Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 123 Asia Pacific Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 124 Asia Pacific Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 125 Asia Pacific Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 126 Asia Pacific Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 127 Asia Pacific Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 128 Asia Pacific Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 129 China Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 130 China Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 131 China Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 132 China Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 133 China Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 134 China Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 135 Japan Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 136 Japan Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 137 Japan Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 138 Japan Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 139 Japan Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 140 Japan Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 141 India Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 142 India Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 143 India Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 144 India Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 145 India Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 146 India Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 147 South Korea Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 148 South Korea Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 149 South Korea Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 150 South Korea Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 151 South Korea Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 152 South Korea Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 153 South-East Asia Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 154 South-East Asia Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 155 South-East Asia Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 156 South-East Asia Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 157 South-East Asia Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 158 South-East Asia Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 159 Rest of Asia Pacific Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 160 Rest of Asia Pacific Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 161 Rest of Asia Pacific Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 162 Rest of Asia Pacific Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 163 Rest of Asia Pacific Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 164 Rest of Asia Pacific Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 165 Latin America Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 166 Latin America Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 167 Latin America Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 168 Latin America Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 169 Latin America Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 170 Latin America Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 171 Brazil Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 172 Brazil Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 173 Brazil Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 174 Brazil Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 175 Brazil Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 176 Brazil Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 177 Argentina Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 178 Argentina Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 179 Argentina Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 180 Argentina Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 181 Argentina Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 182 Argentina Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 183 Rest of Latin America Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 184 Rest of Latin America Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 185 Rest of Latin America Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 186 Rest of Latin America Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 187 Rest of Latin America Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 188 Rest of Latin America Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 189 Middle East Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 190 Middle East Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 191 Middle East Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 192 Middle East Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 193 Middle East Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 194 Middle East Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 195 UAE Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 196 UAE Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 197 UAE Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 198 UAE Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 199 UAE Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 200 UAE Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 201 Saudi Arabia Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 202 Saudi Arabia Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 203 Saudi Arabia Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 204 Saudi Arabia Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 205 Saudi Arabia Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 206 Saudi Arabia Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 207 Rest of Middle East Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 208 Rest of Middle East Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 209 Rest of Middle East Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 210 Rest of Middle East Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 211 Rest of Middle East Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 212 Rest of Middle East Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 213 Africa Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 214 Africa Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 215 Africa Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 216 Africa Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 217 Africa Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 218 Africa Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 219 South Africa Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 220 South Africa Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 221 South Africa Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 222 South Africa Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 223 South Africa Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 224 South Africa Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 225 Egypt Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 226 Egypt Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 227 Egypt Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 228 Egypt Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 229 Egypt Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 230 Egypt Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)
TABLE 231 Rest of Africa Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2019-2023 (US$ Mn)
TABLE 232 Rest of Africa Amyloidosis Therapeutic Treatment Market, by Type of Amyloidosis, 2024-2032 (US$ Mn)
TABLE 233 Rest of Africa Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2019-2023 (US$ Mn)
TABLE 234 Rest of Africa Amyloidosis Therapeutic Treatment Market, by Treatment Modalities, 2024-2032 (US$ Mn)
TABLE 235 Rest of Africa Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2019-2023 (US$ Mn)
TABLE 236 Rest of Africa Amyloidosis Therapeutic Treatment Market, by Disease Severity, 2024-2032 (US$ Mn)