Market Overview
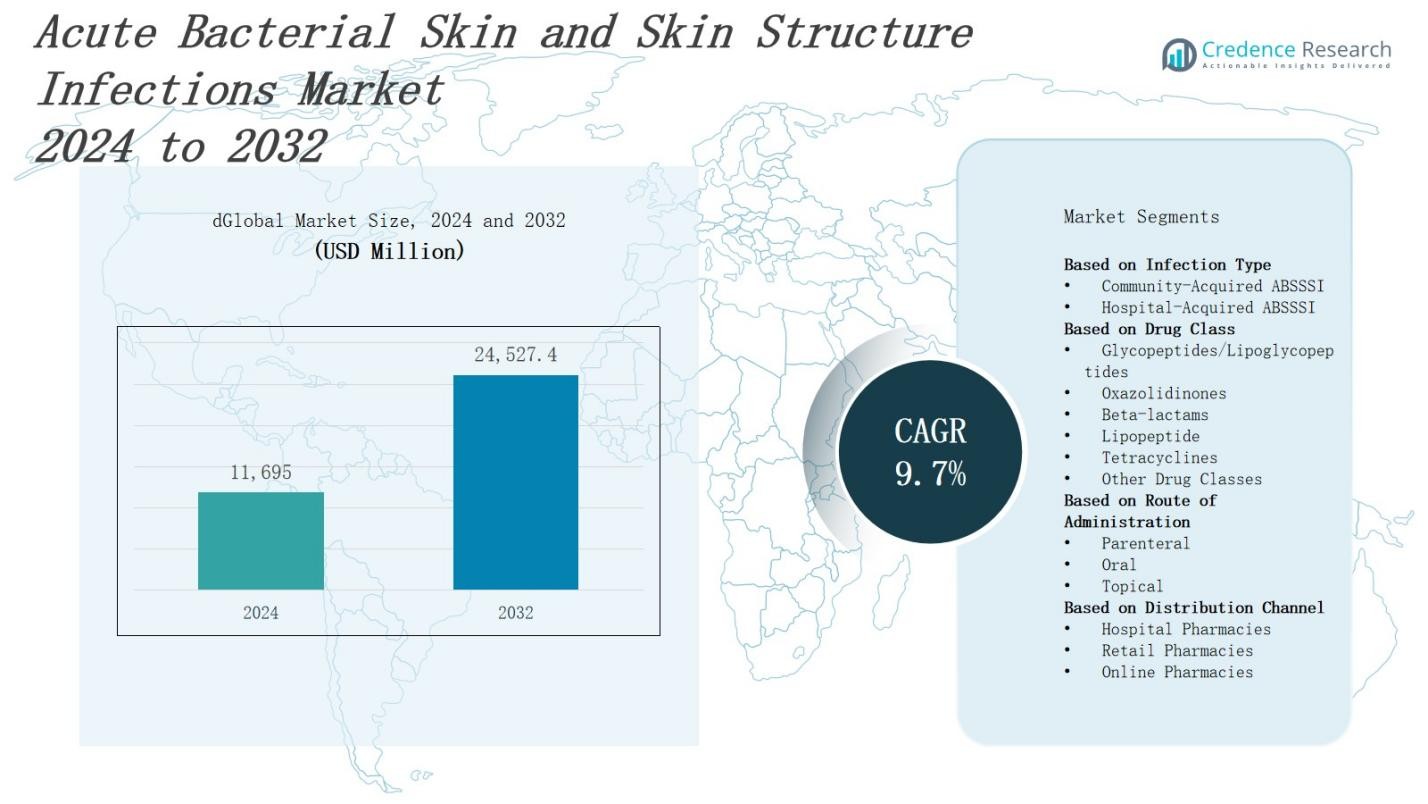
The acute bacterial skin and skin structure infections market is projected to grow from USD 11,695 million in 2024 to USD 24,527.4 million by 2032, registering a compound annual growth rate (CAGR) of 9.7%.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2024 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Acute Bacterial Skin And Skin Structure Infections Market Size 2024 |
USD 11,695 Million |
| Acute Bacterial Skin And Skin Structure Infections Market, CAGR |
9.7% |
| Acute Bacterial Skin And Skin Structure Infections Market Size 2032 |
USD 24,527.4 Million |
The acute bacterial skin and skin structure infections market grows due to rising prevalence of bacterial infections, increasing antibiotic resistance, and a growing geriatric population prone to skin complications. Advances in diagnostic technologies and the introduction of novel antibiotic therapies enhance treatment efficacy, driving market expansion. Additionally, increasing awareness about early diagnosis and treatment, along with rising healthcare expenditure worldwide, supports market growth. Trends include the development of targeted therapies, use of combination treatments to combat resistant strains, and growing adoption of outpatient care and telemedicine for infection management, reflecting the shift toward more efficient and patient-centric healthcare delivery.
The acute bacterial skin and skin structure infections market spans North America, Europe, Asia-Pacific, and the Rest of the World, with North America leading at 35% market share, followed by Europe at 28%, Asia-Pacific at 25%, and the Rest of the World at 12%. Key players driving growth across these regions include AbbVie, Merck, Pfizer, Glenmark Pharmaceuticals, Basilea Pharmaceutica, Cipher Pharmaceuticals, and Menarini Group. These companies focus on innovation, strategic partnerships, and expanding their regional presence to meet diverse market demands and combat antibiotic resistance effectively.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The acute bacterial skin and skin structure infections market is projected to grow from USD 11,695 million in 2024 to USD 24,527 million by 2032, registering a CAGR of 9.7%.
- Rising prevalence of bacterial infections, increasing antibiotic resistance, and a growing geriatric population prone to skin complications drive market growth.
- Advances in diagnostic technologies and novel antibiotic therapies improve treatment efficacy, fueling market expansion globally.
- Growing awareness about early diagnosis, coupled with rising healthcare expenditure worldwide, supports broader market adoption.
- Development of targeted therapies, combination treatments against resistant strains, and growing outpatient care and telemedicine use reflect shifting patient-centric trends.
- North America leads with 35% market share, followed by Europe (28%), Asia-Pacific (25%), and the Rest of the World (12%), driven by varying healthcare infrastructure and infection prevalence.
- Key players such as AbbVie, Merck, Pfizer, Glenmark Pharmaceuticals, Basilea Pharmaceutica, Cipher Pharmaceuticals, and Menarini Group focus on innovation, partnerships, and expanding regional presence to combat resistance effectively.
Market Drivers
Rising Incidence of Bacterial Skin Infections and Antibiotic Resistance
The increasing prevalence of bacterial skin and skin structure infections worldwide drives the market growth significantly. Higher rates of infections such as cellulitis, wound infections, and abscesses create a substantial demand for effective treatments. Concurrently, antibiotic resistance among common pathogens like Staphylococcus aureus and Streptococcus species complicates therapy, prompting the development and adoption of new antibiotics. It accelerates market expansion by necessitating advanced therapeutic options to address resistant strains and improve patient outcomes.
- For instance, Paratek Pharmaceuticals markets Nuzyra (Omadacycline), a novel antibiotic indicated for adult patients with acute bacterial skin and skin structure infections.
Advancements in Antibiotic Development and Diagnostic Technologies
Ongoing innovation in antibiotic research enhances the acute bacterial skin and skin structure infections market. Pharmaceutical companies focus on creating novel antibiotics with improved efficacy against resistant bacteria and better safety profiles. Advances in diagnostic tools facilitate faster and more accurate identification of infection-causing organisms, enabling targeted treatment. These developments improve clinical decision-making and reduce treatment failures, strengthening the demand for advanced therapies in both hospital and outpatient settings.
- For instance, Roche has two novel antibiotics in clinical development specifically targeting carbapenem-resistant Acinetobacter baumannii and other gram-negative bacteria, representing a new class with unprecedented mechanisms of action.
Growing Geriatric Population and Chronic Disease Burden
The expanding elderly population contributes to market growth due to increased vulnerability to skin infections. Age-related immune decline and comorbidities such as diabetes and vascular diseases raise the risk of acute bacterial skin infections. It creates a larger patient pool requiring specialized treatment and management strategies. Chronic wounds and repeated infections in this demographic increase healthcare utilization, boosting demand for effective antibiotic therapies and comprehensive infection care solutions.
Increasing Healthcare Awareness and Expenditure
Rising awareness among healthcare professionals and patients about early diagnosis and proper treatment of skin infections supports market expansion. Governments and private sectors invest in healthcare infrastructure, improving access to advanced therapies. It leads to greater adoption of novel antibiotics and outpatient treatment options, reducing hospital stays and associated costs. Enhanced reimbursement policies and emphasis on antimicrobial stewardship further encourage the use of effective medications, driving steady growth in the acute bacterial skin and skin structure infections market.
Market Trends
Expansion of Targeted Antibiotic Therapies and Combination Treatments
The acute bacterial skin and skin structure infections market increasingly shifts toward targeted antibiotic therapies designed to address specific resistant bacteria. Pharmaceutical companies focus on developing drugs that precisely inhibit problematic pathogens, reducing off-target effects and improving patient outcomes. Combination treatments gain popularity to enhance efficacy against multidrug-resistant strains by utilizing synergistic drug actions. It enables clinicians to tailor therapies based on pathogen susceptibility, optimizing infection management and minimizing resistance development over time.
- For instance, Summit Therapeutics developed ridinilazole, a targeted antibiotic shown to treat Clostridioides difficile infections by preserving beneficial gut microbiota, thus reducing collateral damage compared to broad-spectrum drugs.
Integration of Rapid Diagnostic Technologies for Early Detection
Rapid diagnostic tools gain traction in the acute bacterial skin and skin structure infections market to accelerate pathogen identification and antibiotic susceptibility testing. These technologies provide clinicians with timely information, allowing for prompt initiation of appropriate therapies. It reduces empirical antibiotic use and limits unnecessary exposure to broad-spectrum drugs, helping control antibiotic resistance. Point-of-care testing devices become more prevalent in outpatient settings, supporting efficient patient management and reducing hospitalization duration.
- For instance, T2Bacteria® Panel by T2 Biosystems provides identification of five key bloodstream infection bacteria within 3-5 hours, enabling quicker targeted therapy and reduced broad-spectrum antibiotic use.
Growth in Outpatient and Home-Based Care Models
The market experiences a trend toward outpatient antibiotic therapies and home-based infection management for suitable cases. Shifting care from hospitals to outpatient settings decreases healthcare costs and improves patient comfort. It facilitates earlier discharge by enabling intravenous or oral antibiotic administration at home under professional supervision. This trend responds to healthcare system pressures and advances in drug formulations that support flexible administration, contributing to broader market penetration and improved access to care.
Focus on Antimicrobial Stewardship and Regulatory Support
The acute bacterial skin and skin structure infections market benefits from increasing emphasis on antimicrobial stewardship programs that promote rational antibiotic use. Hospitals and healthcare providers implement guidelines to optimize treatment regimens, reduce misuse, and slow resistance emergence. It encourages development and adoption of novel antibiotics through regulatory incentives like expedited approvals and extended exclusivity periods. These initiatives stimulate innovation while safeguarding existing therapies, ensuring sustainable market growth and improved patient outcomes.
Market Challenges Analysis
Rising Antibiotic Resistance and Limited Novel Drug Development
The acute bacterial skin and skin structure infections market faces significant challenges from increasing antibiotic resistance among key bacterial pathogens. Resistant strains reduce the effectiveness of existing treatments, leading to higher rates of treatment failure and prolonged infections. The slow pace of novel antibiotic development limits options available to clinicians, creating gaps in effective therapy. It forces healthcare providers to rely on older drugs with potential side effects or less efficacy. This challenge also increases the economic burden on healthcare systems due to longer hospital stays and additional treatments required for resistant infections.
Regulatory Hurdles and High Treatment Costs
Stringent regulatory requirements for antibiotic approval complicate market entry for new drugs, delaying availability. The lengthy and costly clinical trial processes discourage investment in antibiotic research and development. It restricts the introduction of innovative therapies necessary to combat evolving bacterial threats. High treatment costs and limited reimbursement policies in certain regions further constrain patient access to advanced antibiotics. These factors collectively hinder market growth and affect the adoption of new therapeutic options, presenting ongoing obstacles for the acute bacterial skin and skin structure infections market.
Market Opportunities
Development of Novel Antibiotics and Innovative Drug Delivery Systems
The acute bacterial skin and skin structure infections market presents significant opportunities for developing novel antibiotics that effectively target resistant bacteria. Pharmaceutical companies can focus on breakthrough therapies with unique mechanisms of action to overcome current treatment limitations. Innovations in drug delivery, such as long-acting formulations and topical applications, offer enhanced patient compliance and improved therapeutic outcomes. It opens avenues for personalized treatment approaches tailored to infection severity and patient needs. Expanding research collaborations and leveraging advanced biotechnology can accelerate product pipelines, addressing unmet clinical demands and driving market growth.
Expansion into Emerging Markets and Telemedicine Integration
Emerging economies provide promising opportunities for the acute bacterial skin and skin structure infections market due to rising infection prevalence and increasing healthcare infrastructure investments. It can benefit from growing awareness about early diagnosis and treatment availability in these regions. Integrating telemedicine and digital health platforms facilitates remote patient monitoring and consultation, improving access to timely care and reducing hospital burden. These technological advances enable better management of skin infections in underserved areas. Expanding market presence through strategic partnerships and localized solutions can capitalize on evolving healthcare trends and broaden the customer base.
Market Segmentation Analysis:
By Infection Type
The acute bacterial skin and skin structure infections market segments into community-acquired and hospital-acquired infections. Community-acquired ABSSSI accounts for a larger share due to higher incidence rates outside healthcare settings. Hospital-acquired ABSSSI presents challenges related to multidrug-resistant pathogens, driving demand for advanced antibiotics. It requires specialized treatment approaches to manage infections acquired during hospital stays, contributing to the overall market growth by addressing critical care needs.
- For instance, Paladin Labs, a subsidiary of Endo International, introduced Xydalba (dalbavancin) in Canada, which provides a convenient single or two-dose intravenous therapy for community-acquired ABSSSI, improving outpatient treatment options.
By Drug Class
The market includes multiple drug classes such as glycopeptides/lipoglycopeptides, oxazolidinones, beta-lactams, lipopeptides, tetracyclines, and others. Beta-lactams dominate due to their broad-spectrum activity and established clinical use. Glycopeptides and lipoglycopeptides gain traction for treating resistant infections. It promotes continuous innovation in drug development to improve efficacy and safety, supporting market expansion across diverse therapeutic options.
- For instance, dalbavancin, a second-generation lipoglycopeptide derived from teicoplanin, features a lipophilic side chain that enhances potency and extends its half-life, improving treatment against resistant bacteria.
By Route of Administration
Parenteral administration holds a significant share due to its rapid and effective delivery in severe infections. Oral antibiotics offer convenience and support outpatient treatment, enhancing patient compliance. Topical drugs provide localized therapy for mild infections. It benefits from this varied administration landscape, enabling tailored treatment strategies suited to infection severity and patient preferences.
Segments:
Based on Infection Type
- Community-Acquired ABSSSI
- Hospital-Acquired ABSSSI
Based on Drug Class
- Glycopeptides/Lipoglycopeptides
- Oxazolidinones
- Beta-lactams
- Lipopeptide
- Tetracyclines
- Other Drug Classes
Based on Route of Administration
Based on Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Based on the Geography:
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis
North America
North America holds the largest share in the acute bacterial skin and skin structure infections market, accounting for 35% of the global revenue. The region benefits from advanced healthcare infrastructure, high prevalence of skin infections, and strong adoption of novel antibiotics. It drives market growth through substantial R&D investments and regulatory support for innovative drug approvals. Increasing awareness about antimicrobial resistance and antimicrobial stewardship programs further enhances treatment quality. The presence of leading pharmaceutical companies and well-established distribution networks strengthens market penetration and accessibility.
Europe
Europe accounts for 28% of the acute bacterial skin and skin structure infections market share. The region experiences steady growth supported by rising cases of hospital-acquired infections and a growing geriatric population. Healthcare reforms and stringent infection control policies improve patient outcomes and stimulate demand for effective antibiotics. It benefits from government initiatives promoting rational antibiotic use and the introduction of advanced diagnostic tools. High healthcare expenditure and expanding outpatient care contribute to broader market adoption across European countries.
Asia-Pacific
Asia-Pacific holds 25% of the market share and exhibits the fastest growth due to increasing infection rates and expanding healthcare access. The rising population, urbanization, and improved disease awareness drive demand for acute bacterial skin infection treatments. It faces challenges from widespread antibiotic misuse, which accelerates resistance, creating a need for novel therapies. Government efforts to strengthen healthcare infrastructure and expand insurance coverage support market expansion. Growing pharmaceutical manufacturing capabilities in countries like India and China also enhance regional supply chains.
Rest of the World
The Rest of the World region contributes 12% to the acute bacterial skin and skin structure infections market. Emerging economies in Latin America, the Middle East, and Africa show increasing prevalence of skin infections due to limited healthcare access and rising chronic disease rates. It leverages growing investments in healthcare infrastructure and awareness campaigns to improve treatment rates. Market growth depends on improving distribution channels and affordable antibiotic availability. International collaborations and donor-funded programs also play roles in expanding care reach in these regions.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Cipher Pharmaceuticals
- Glenmark Pharmaceuticals
- Menarini Group
- Paratek Pharmaceuticals
- Merck
- AbbVie
- Basilea Pharmaceutica
- Melinta Therapeutics
- Pfizer
- Nabriva Therapeutics
- Sandoz
- Endo Pharmaceuticals
Competitive Analysis
The acute bacterial skin and skin structure infections market features intense competition among global pharmaceutical companies focused on developing innovative antibiotic therapies. Leading players like Sandoz, Paratek Pharmaceuticals, AbbVie, Pfizer and Glenmark Pharmaceuticals invest heavily in research and development to address rising antibiotic resistance and expand their product portfolios. It benefits from strategic collaborations, acquisitions, and licensing agreements that enhance technological capabilities and market reach. Companies emphasize launching novel drugs with improved efficacy and safety profiles to capture greater market share. Strong distribution networks and regulatory expertise provide competitive advantages in securing approvals and timely market entry. Cost-effectiveness and patient compliance remain key factors influencing product adoption. The market’s competitive landscape drives continuous innovation and price competition, pushing companies to optimize supply chains and invest in marketing efforts. This dynamic environment fosters development of advanced treatments tailored to both community-acquired and hospital-acquired infections, ensuring responsiveness to evolving clinical needs and sustaining long-term growth.
Recent Developments
- In July 2023, Melinta Therapeutics partnered with the U.S. government’s BARDA to advance two FDA-approved antibiotics, Baxdela and Minocin, for pediatric patients, expanding their pipeline in infectious disease treatment relevant to ABSSSI.
- In 2025, 3D Systems introduced the Figure 4® 135 3D printer and Figure 4 Tough 75C FR Black material at RAPID+TCT, targeting high-mix, low-volume applications such as motorsports components and furniture hardware.
- In 2024, Colibrium Additive, a GE Aerospace company, unveiled the Spectra M electron beam melting 3D printer designed for medical and orthopedic implant manufacturing, offering reduced cost-per-part and production expenses.
- In 2025, HP launched the HP 3D High Reusability PA 12 FR material, developed with Evonik, featuring 60% reusability that lowers total ownership costs by 20% and reduces carbon emissions by 10%, while meeting strict safety standards.
Market Concentration & Characteristics
The acute bacterial skin and skin structure infections market exhibits a moderately concentrated competitive landscape dominated by several global pharmaceutical leaders. It features a mix of large multinational corporations and specialized biotech firms that drive innovation through extensive research and development efforts. The market’s characteristics include high entry barriers due to stringent regulatory requirements, significant investment in clinical trials, and the critical need for novel antibiotics to combat rising resistance. Companies focus on developing drugs with enhanced efficacy and safety profiles to address both community-acquired and hospital-acquired infections. It emphasizes strategic partnerships, acquisitions, and licensing agreements to expand product portfolios and geographic reach. The market balances established therapies with emerging treatments that target resistant pathogens, reflecting ongoing adaptation to evolving clinical challenges. This dynamic fosters sustained growth and competitive differentiation based on innovation, regulatory compliance, and access to diverse healthcare markets worldwide.
Report Coverage
The research report offers an in-depth analysis based on Infection Type, Drug Class, Route of Administration, Distribution Channel and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market will benefit from ongoing development of novel antibiotics targeting resistant bacteria.
- Increased adoption of rapid diagnostic tools will enable earlier and more accurate infection management.
- Expansion of outpatient care and home-based treatment will improve patient convenience and reduce healthcare costs.
- Growing geriatric populations will create higher demand for specialized infection therapies.
- Pharmaceutical companies will focus on combination therapies to enhance effectiveness against multidrug-resistant strains.
- Regulatory agencies will continue to support expedited approvals for innovative antibacterial drugs.
- Telemedicine integration will enhance access to care and monitoring for skin infections.
- Emerging markets will drive growth due to rising infection prevalence and improving healthcare infrastructure.
- Antimicrobial stewardship programs will promote rational antibiotic use, preserving treatment efficacy.
- Strategic collaborations and mergers will accelerate innovation and expand global market reach.




