Market Overview:
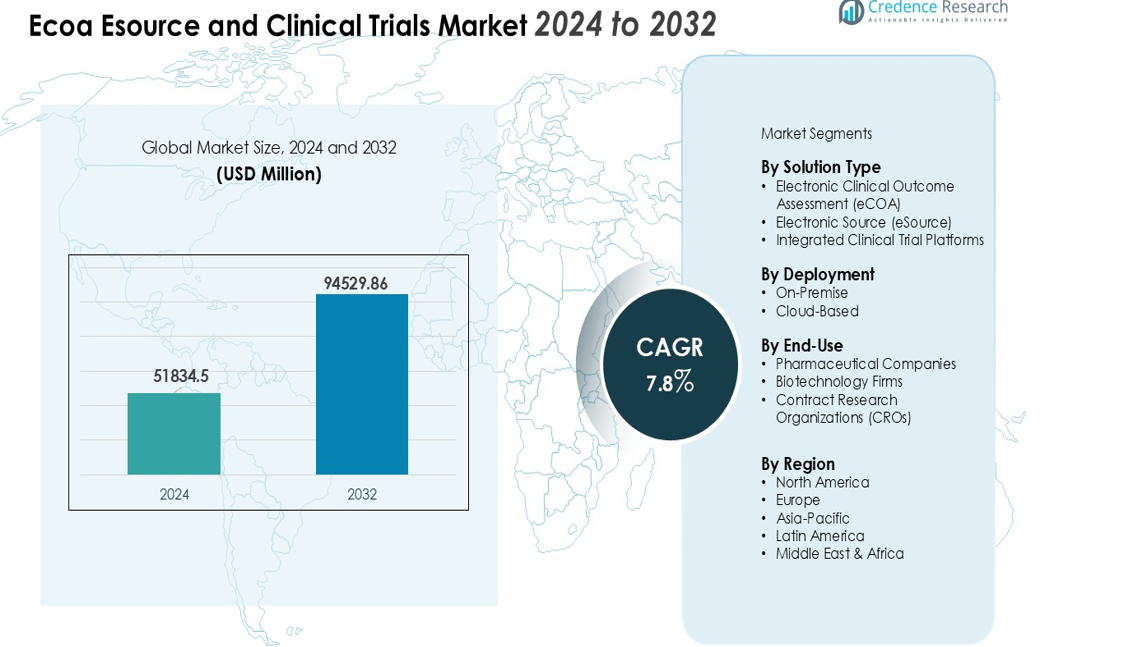
The Ecoa Esource and Clinical Trials Market size was valued at USD 51834.5 million in 2024 and is anticipated to reach USD 94529.86 million by 2032, at a CAGR of 7.8% during the forecast period (2024-2032).
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| eCOA, eSource and Clinical Trials Market Size 2024 |
USD 51834.5 million |
| eCOA, eSource and Clinical Trials Market, CAGR |
7.8% |
| eCOA, eSource and Clinical Trials Market Size 2032 |
USD 94529.86 million |
Key market drivers include the rising volume and complexity of clinical trials worldwide, which necessitates streamlined electronic data collection, real-time monitoring, and secure data management. The shift toward decentralized and patient-centric clinical trials, enabled by remote monitoring, wearable devices, mobile health applications, and cloud-based electronic platforms, has further accelerated the adoption of eCOA and eSource solutions. Additionally, regulatory encouragement for electronic clinical outcome assessments and the growing need for data integrity, transparency, and efficiency are reinforcing market expansion.
Regionally, North America leads the market, supported by advanced pharmaceutical R&D infrastructure, early technology adoption, and favorable regulatory frameworks. Meanwhile, Asia-Pacific is emerging as the fastest-growing region due to increasing clinical research activities, outsourcing of trials, and the adoption of digital trial management solutions, presenting significant growth opportunities for the market.
Market Insights:
- The Ecoa Esource and Clinical Trials Market was valued at USD 51,834.5 million in 2024 and is projected to reach USD 94,529.86 million by 2032, growing at a CAGR of 7.8%.
- Rising complexity and volume of global clinical trials drive demand for digital solutions that enable real-time data capture, reduce errors, and ensure protocol compliance.
- Decentralized and patient-centric trials are accelerating adoption of eCOA and eSource solutions, supported by wearable devices, mobile health apps, and remote monitoring platforms.
- Regulatory support for electronic clinical outcome assessments enhances market adoption, enabling accurate, traceable, and auditable trial data.
- Focus on data integrity, transparency, and operational efficiency motivates pharmaceutical and biotechnology companies to adopt integrated digital trial management platforms.
- High implementation costs, complex integration with legacy systems, and stringent data privacy requirements pose challenges to widespread adoption.
- Regionally, North America leads with 42% market share due to advanced R&D infrastructure, Europe holds 28% supported by regulatory harmonization, and Asia-Pacific represents 20% and grows fastest due to increasing clinical research activities and outsourcing.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers:
Rising Complexity and Volume of Clinical Trials Driving Digital Solution Adoption
The increasing complexity and volume of clinical trials globally has created a strong need for digital solutions. Sponsors require accurate, real-time data collection to ensure trial efficiency and patient safety. The eCOA, eSource and Clinical Trials Market benefits from its ability to streamline data management across multiple sites. It reduces errors in data entry and accelerates protocol adherence. This efficiency helps organizations meet regulatory requirements while managing larger and more intricate studies.
- For instance, Medable, a pioneer in decentralized clinical trials, integrates telehealth, ePRO, and remote monitoring technologies, enabling real-time data access and collaboration across sites, which helped reduce site visits by 30% in recent studies.
Shift Toward Decentralized and Patient-Centric Clinical Trials Enhancing Market Demand
Clinical trials are moving toward decentralized models, focusing on patient convenience and remote participation. It enables data capture from wearable devices, mobile health applications, and home-based monitoring systems. The eCOA, eSource and Clinical Trials Market supports this transformation by providing secure, real-time access to patient-reported outcomes. Organizations gain flexibility and better engagement with participants. This trend increases the demand for digital clinical trial platforms capable of remote monitoring.
- For Instance, Science 37, its patient recruitment solution enabled customers to enroll 42% of a trial cohort in eight weeks for a rare disease study. The company also reports its technology platform accelerates enrollment up to 15 times faster than traditional methods, delivering 100% medically qualified referrals.
Regulatory Encouragement for Electronic Clinical Outcome Assessments Driving Implementation
Regulatory agencies are increasingly supporting electronic methods for clinical outcome assessments. It improves the accuracy, traceability, and auditability of trial data. Compliance with these regulations is critical for global trial approvals. The eCOA, eSource and Clinical Trials Market provides standardized electronic solutions to satisfy these requirements. Companies benefit from faster regulatory review and reduced risk of data discrepancies.
Growing Focus on Data Integrity, Transparency, and Operational Efficiency
Pharmaceutical and biotechnology companies prioritize data integrity and operational efficiency in clinical trials. It ensures reliable results and maintains sponsor credibility. The eCOA, eSource and Clinical Trials Market enables consistent data capture, secure storage, and streamlined reporting. Organizations reduce manual errors and save time during analysis. This focus on high-quality, transparent data continues to drive adoption of digital trial management solutions.
Market Trends:
Integration of Advanced Digital Technologies Enhancing Clinical Trial Efficiency
The eCOA, eSource and Clinical Trials Market shows a strong trend toward integrating advanced digital technologies, including cloud computing, artificial intelligence, and machine learning. It enables sponsors to analyze large volumes of clinical data in real-time and identify patterns that support faster decision-making. Remote patient monitoring devices and mobile applications provide continuous data streams directly to trial databases. It reduces operational delays and improves protocol compliance across multiple study sites. Organizations increasingly rely on these technologies to enhance trial quality and minimize costs. Data visualization tools and automated reporting features help stakeholders monitor trial progress effectively.
- For example, the WeTrials platform enables remote patient access and provides educational resources and personalized guidance to help participants find and navigate clinical trials, demonstrating an effort to enhance patient engagement and streamline operations in multi-site studies.
Expansion of Decentralized Trials and Focus on Patient-Centric Approaches
Decentralized clinical trials continue to gain momentum, emphasizing patient convenience and remote participation. The eCOA, eSource and Clinical Trials Market supports these trials by providing secure electronic data capture and management solutions. It enables direct patient input through mobile devices, wearable sensors, and home-based monitoring, ensuring real-time access for investigators. This approach improves patient engagement and retention while maintaining data integrity. Sponsors adopt platforms that allow integration of multiple data sources for a holistic view of trial outcomes. Digital trial platforms facilitate faster recruitment and broaden access to diverse patient populations. The market trend reflects a sustained move toward more flexible, technology-driven, and patient-focused clinical research strategies.
- For instance, Science 37 builds global decentralized trial networks facilitating diverse patient recruitment, leading to broader access and improved outcome robustness.
Market Challenges Analysis:
High Implementation Costs and Complex Integration Hindering Adoption
The eCOA, eSource and Clinical Trials Market faces challenges from high implementation costs and the complexity of integrating digital solutions into existing clinical workflows. It requires significant investment in technology infrastructure, staff training, and ongoing maintenance. Smaller organizations often find adoption financially burdensome. Data migration from legacy systems can delay deployment and disrupt ongoing trials. Compatibility issues with diverse electronic systems may affect seamless data flow. These barriers slow widespread adoption despite the benefits of enhanced efficiency and data accuracy.
Data Privacy, Regulatory Compliance, and Security Concerns Limiting Growth
Data privacy and regulatory compliance remain critical challenges for the eCOA, eSource and Clinical Trials Market. It must adhere to strict standards for patient confidentiality, data protection, and cross-border information transfer. Any security breach can undermine trust and lead to regulatory penalties. Variations in regulations across regions complicate trial management for multinational studies. Organizations must invest in robust cybersecurity measures and compliance protocols. Managing large volumes of sensitive clinical data while maintaining accuracy and accessibility adds operational complexity. These challenges constrain market growth and require strategic planning for secure and compliant implementation.
Market Opportunities:
Expansion of Decentralized and Remote Clinical Trials Creating Growth Potential
The eCOA, eSource and Clinical Trials Market presents significant opportunities through the expansion of decentralized and remote clinical trials. It enables sponsors to reach wider patient populations and reduce geographical barriers in trial recruitment. Digital platforms support real-time data capture from wearable devices, mobile apps, and home-based monitoring systems. Organizations can enhance patient engagement and retention while maintaining high-quality data. Integration of multiple data sources allows comprehensive analysis of trial outcomes. The growing demand for flexible, patient-centric trial designs offers sustained market potential.
Adoption of Advanced Analytics and Artificial Intelligence Enhancing Market Prospects
Increasing adoption of advanced analytics, artificial intelligence, and machine learning offers additional opportunities for the eCOA, eSource and Clinical Trials Market. It allows efficient processing of large volumes of clinical data and identification of predictive insights. Sponsors can optimize trial design, monitor patient safety, and accelerate decision-making. Automation of reporting and risk management improves operational efficiency. Growing interest in real-world evidence and digital biomarkers supports further technology integration. These advancements position the market for continued growth and innovation in clinical research.
Market Segmentation Analysis:
By Solution Type
The eCOA, eSource and Clinical Trials Market is segmented by solution type into electronic clinical outcome assessment (eCOA), electronic source (eSource), and integrated clinical trial platforms. It allows organizations to select solutions based on trial complexity and data requirements. eCOA solutions dominate adoption due to their ability to capture patient-reported outcomes efficiently and maintain data integrity. eSource platforms support direct data capture from electronic medical records, reducing manual errors and accelerating trial timelines. Integrated platforms provide end-to-end trial management, combining data collection, monitoring, and reporting into a unified system. These solutions enhance operational efficiency and improve overall trial quality.
- For instance, the ICON Digital Platform integrates eConsent, eCOA, eSource, and televisit modules to facilitate decentralized clinical trials, resulting in a reported 20% improvement in patient engagement and a 15% reduction in trial operational complexities.
By Deployment Mode
Deployment in the eCOA, eSource and Clinical Trials Market includes on-premise and cloud-based solutions. It offers flexibility to meet the specific infrastructure and security needs of different organizations. Cloud-based deployment leads the market due to its scalability, remote accessibility, and reduced IT management costs. On-premise deployment remains relevant for organizations requiring complete control over data storage and internal systems. Both deployment types enable real-time data access and support decentralized trial models. Adoption depends on trial size, data sensitivity, and organizational IT capabilities.
- For example, Castor eCOA platform achieves 90% of user acceptance testing completion within 4 weeks, maintaining robust validation in a modern, cloud-native environment.
By End-Use
End-use segments include pharmaceutical companies, biotechnology firms, and contract research organizations (CROs). It helps sponsors manage complex trials, ensure regulatory compliance, and accelerate drug development. Pharmaceutical companies hold the largest share due to high R&D investments and the need for efficient digital solutions. CROs increasingly adopt these platforms to provide scalable services to multiple sponsors. Biotechnology firms leverage eCOA and eSource solutions to enhance patient-centric trials and improve data accuracy. The market growth aligns with expanding clinical research activities across these end-users.
Segmentations:
By Solution Type
- Electronic Clinical Outcome Assessment (eCOA)
- Electronic Source (eSource)
- Integrated Clinical Trial Platforms
By Deployment
By End-Use
- Pharmaceutical Companies
- Biotechnology Firms
- Contract Research Organizations (CROs)
Region
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
North America Leading Adoption Due to Advanced Pharmaceutical Infrastructure
North America accounts for 42% of the global eCOA, eSource and Clinical Trials Market, reflecting its leadership in clinical research and digital trial technologies. The region maintains a strong presence of major pharmaceutical companies and technology providers. It benefits from well-established research infrastructure and regulatory frameworks that encourage electronic clinical outcome assessments. It enables efficient data capture, storage, and reporting across multiple trial sites. High investment in clinical research and early adoption of innovative trial platforms reinforce its market position. Sponsors rely on these solutions to ensure compliance, improve patient engagement, and enhance operational efficiency.
Europe Driving Growth Through Regulatory Support and Technology Integration
Europe holds 28% of the global eCOA, eSource and Clinical Trials Market, supported by harmonized regulatory standards and growing adoption of digital clinical solutions. The region facilitates cross-border collaboration for multinational studies and promotes standardized electronic platforms. It allows sponsors to integrate remote patient monitoring, real-time reporting, and centralized data management. Investments in patient-centric and decentralized trials further enhance operational efficiency. It strengthens trial quality, accelerates study timelines, and supports diverse therapeutic areas across the continent.
Asia-Pacific Emerging as the Fastest-Growing Region with Expanding Clinical Research
Asia-Pacific represents 20% of the global eCOA, eSource and Clinical Trials Market and shows the highest growth rate among all regions. Rapid expansion of clinical research, outsourcing of trials, and increasing pharmaceutical manufacturing drive adoption. It provides access to large and diverse patient populations, enabling cost-effective trial execution. Digital platforms support real-time data collection, remote monitoring, and streamlined reporting. Government initiatives promoting technology adoption and data security strengthen market development. The region offers substantial opportunities for long-term growth and innovation in clinical trials.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- Medidata Solutions, Inc.
- Oracle Corporation
- Veeva Systems Inc.
- Parexel International Corporation
- CRF Health (now part of Medidata)
- BioClinica, Inc.
- ERT (eResearchTechnology)
- ICON plc
- IQVIA Holdings, Inc.
- Signant Health
- YPrime, LLC
- ClinOne
Competitive Analysis:
The eCOA, eSource and Clinical Trials Market is highly competitive, driven by the presence of established technology providers and contract research organizations offering advanced digital solutions. Key players focus on product innovation, strategic partnerships, and geographic expansion to strengthen market positions. It emphasizes integration of cloud-based platforms, AI-driven analytics, and patient-centric tools to differentiate offerings. Companies invest in scalable solutions that support decentralized trials, real-time data capture, and regulatory compliance. Competitive strategies include acquisitions, collaborations with pharmaceutical sponsors, and enhancement of service portfolios to provide end-to-end trial management. Market leaders prioritize client support and training to ensure seamless adoption of their platforms. Smaller firms leverage niche solutions or specialized services to capture targeted market segments. Overall, competition encourages continuous innovation, improved operational efficiency, and enhanced trial quality across the global market.
Recent Developments:
- In October 2025, Medidata Solutions, Inc. expanded its partnership with Sanofi to deepen collaboration on AI-enabled clinical development.
- In March 2025, ICON plc formed a partnership with Mural Health Technologies, Inc. to improve clinical trial participant and site experience, utilizing Mural Link’s platform for payments, travel coordination, and communication, facilitating patient-centric trial management and reducing participation barriers.
Report Coverage:
The research report offers an in-depth analysis based on Solution Type, Deployment, End-Use and Region. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and ITALY economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook:
- Increasing adoption of decentralized and patient-centric clinical trials will expand market growth.
- Integration of artificial intelligence and advanced analytics will enhance data-driven decision-making.
- Cloud-based platforms will dominate deployment due to scalability, flexibility, and remote access.
- Expansion into emerging markets, especially in Asia-Pacific, will create new growth opportunities.
- Regulatory support for electronic clinical outcome assessments will accelerate digital solution adoption.
- Enhanced focus on real-time monitoring and data integrity will drive investment in eCOA and eSource solutions.
- Collaboration between technology providers and pharmaceutical companies will improve trial efficiency.
- Growing demand for wearable devices and mobile health applications will strengthen digital trial capabilities.
- Development of integrated platforms offering end-to-end trial management will gain traction.
- Continuous innovation in automation, reporting, and compliance tools will sustain long-term market expansion.




