Market Overview:
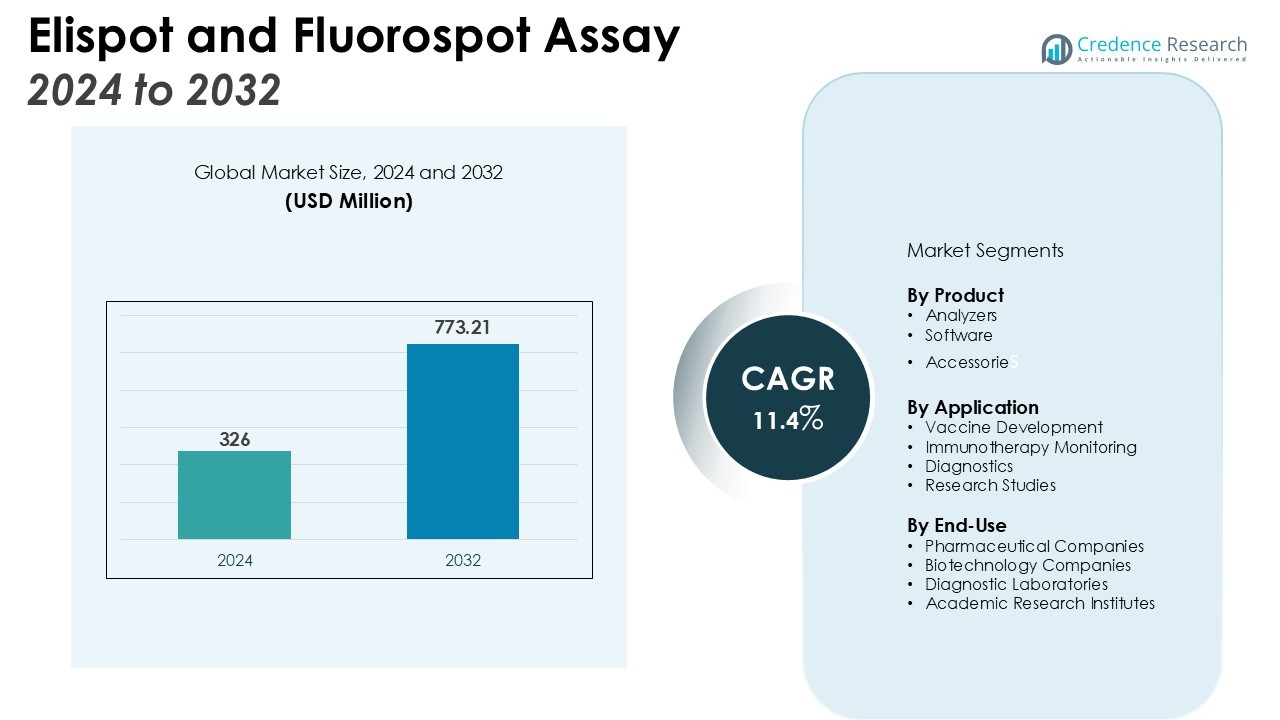
The Elispot and Fluorospot Assay Market size was valued at USD 326 million in 2024 and is anticipated to reach USD 773.21 million by 2032, at a CAGR of 11.4% during the forecast period (2024-2032).
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Elispot and Fluorospot Assay Market Size 2024 |
USD 326 Million |
| Elispot and Fluorospot Assay Market, CAGR |
11.4% |
| Elispot and Fluorospot Assay Market Size 2032 |
USD 773.21 Million |
The ELISpot and FluoroSpot assay market is growing steadily, driven by increasing demand for advanced immune monitoring tools. Rising prevalence of chronic and infectious diseases, combined with the expansion of vaccine development and immunotherapy research, is fueling adoption. Continuous innovations such as multiplexing platforms, automated systems, and AI-enabled analysis are enhancing sensitivity and efficiency, encouraging broader use in clinical and research settings. Key end users include hospitals, diagnostic laboratories, pharmaceutical manufacturers, and academic institutions, all of which benefit from reliable, high-performance assays that support critical immunological insights.
The market shows strong presence in North America, where advanced healthcare systems and significant research investments continue to support widespread utilization. The region also benefits from robust infrastructure and well-established diagnostic networks. In Asia-Pacific, growth momentum is accelerating due to rising healthcare needs, expanding clinical trial activity, and improving access to modern diagnostic tools. Meanwhile, Europe remains an important contributor, with growing adoption in immunology research and diagnostic applications supported by favorable healthcare initiatives. This global mix of established and rapidly developing regions underlines the broad and sustained potential of the ELISpot and FluoroSpot assay market, ensuring consistent opportunities for expansion across diverse healthcare landscapes.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights:
- The Elispot and Fluorospot Assay Market was valued at USD 326 million and is projected to reach USD 773.21 million by 2032, driven by steady double-digit growth.
- Demand is fueled by rising cases of chronic and infectious diseases alongside expanding vaccine and immunotherapy pipelines.
- Automation, multiplexing platforms, and AI-driven analysis are enhancing precision, throughput, and efficiency across research and diagnostic workflows.
- Key end users include hospitals, diagnostic laboratories, pharmaceutical companies, and academic institutions seeking reliable immune monitoring solutions.
- North America leads the market with 42% share, supported by advanced infrastructure, research investments, and robust industry-academia collaborations.
- Asia-Pacific holds 29% share and shows the fastest growth, backed by rising healthcare needs, expanding trials, and government initiatives.
- Europe accounts for 21% share, supported by strong research programs, regulatory frameworks, and adoption in translational medicine and diagnostics.
Market Drivers:
Rising Focus on High-Throughput Immunological Analysis
The Elispot and Fluorospot Assay Automation Market is expanding due to the demand for high-throughput testing. Researchers and laboratories require faster, more precise methods to process large sample volumes. Automated platforms reduce manual errors and increase reproducibility, making them highly valuable in clinical and research environments. This shift toward automation supports both large-scale clinical trials and routine diagnostic workflows, positioning the technology as a critical enabler of advanced immunological research.
- For instance, the AID iSpot Robot from Autoimmun Diagnostika is a high-throughput system that can process up to 30 plates in a single automated run, completing the analysis in under 90 minutes.
Growing Demand for Standardization and Reproducibility in Assays
Automation addresses long-standing concerns about variability in manual assays. The Elispot and Fluorospot Assay Automation Market benefits from systems that ensure consistent sample handling, uniform assay execution, and reliable data capture. Standardized results are essential for regulatory approval processes, especially in vaccine development and immunotherapy research. By offering precise reproducibility, automated solutions help institutions maintain compliance while delivering results with high scientific integrity.
Expanding Applications in Vaccine and Immunotherapy Development
The Elispot and Fluorospot Assay Automation Market is supported by the growing scope of vaccine pipelines and cell-based immunotherapies. Automated assays enable efficient evaluation of immune responses, reducing turnaround times for critical studies. Pharmaceutical and biotechnology companies integrate these systems to monitor T-cell and B-cell activity with greater accuracy. This capability strengthens their ability to accelerate development timelines and bring therapies to market faster.
Integration of Digital Tools and Advanced Imaging Technologies
Digital analysis platforms and enhanced imaging modules are integral drivers for the Elispot and Fluorospot Assay Automation Market. Automation combined with AI-driven imaging improves sensitivity and detection of cytokine-secreting cells. It enables multiparametric analysis, allowing laboratories to extract deeper insights from immune responses. These advancements make automated systems indispensable for modern immunological research, creating sustained demand across clinical, academic, and industrial sectors.
- For instance, the AID iSpot Spectrum analyzer features a 7&1 filter wheel (allowing for up to 7 fluorescent filters), along with a high-resolution camera, enabling detailed analysis of both enzymatic and fluorescent assays on 96- and 384-well plates.
Market Trends:
Adoption of Multiplexing and Digital Advancements in Immune Monitoring
The Elispot and Fluorospot Assay Automation Market is witnessing strong momentum through the integration of multiplexing capabilities and digital technologies. Laboratories are moving toward platforms that can detect multiple cytokines simultaneously, offering a more comprehensive view of immune responses. It supports advanced research in vaccine development, immunotherapies, and chronic disease studies where detailed immune profiling is critical. AI-driven imaging and automated analysis software enhance accuracy, speed, and data interpretation, reducing human error in high-volume workflows. The adoption of cloud-based platforms is further enabling remote data access, collaboration across research institutions, and integration with clinical trial management systems. This trend is strengthening the role of automation in ensuring scalability and reliability for both academic and industrial applications.
- For instance, Mabtech’s EYRAplex™ Human Cytokine kit is a bead-based multiplex assay that enables the simultaneous detection and quantification of 29 different human cytokines in a single sample.
Increasing Use in Translational Research and Personalized Medicine
The Elispot and Fluorospot Assay Automation Market is increasingly aligned with translational research and personalized healthcare initiatives. It plays a key role in bridging laboratory findings with clinical applications, enabling researchers to assess immune responses at the individual patient level. Automated platforms support the development of biomarker-driven therapies by providing accurate and reproducible data. The rise of personalized medicine requires immune monitoring tools that deliver consistent results across diverse patient populations. Automation ensures that clinical studies generate robust data sets, supporting regulatory submissions and accelerating therapeutic approvals. Growing emphasis on patient-specific immunological profiling is expected to sustain this trend, making automated assays a standard in modern healthcare and biomedical research.
- For instance, Mabtech’s IRIS 2 automated reader enhances multiplexing capabilities in this field. The system can simultaneously analyze up to four different analytes from a single well, allowing for a more detailed characterization of immune cell responses in one pass.
Market Challenges Analysis:
High Implementation Costs and Technical Complexity
The Elispot and Fluorospot Assay Automation Market faces challenges linked to high initial investment and complex system integration. Advanced automated platforms require substantial capital, limiting adoption among smaller laboratories and academic institutions. It often demands skilled personnel to manage installation, calibration, and operation, creating additional resource constraints. Limited budgets in developing regions further restrict the adoption of automation, despite growing demand for immunological assays. Maintenance and upgrades add to long-term expenses, making return on investment a critical concern for end users. These financial and technical barriers continue to slow the pace of widespread automation adoption.
Regulatory Compliance and Data Standardization Issues
The Elispot and Fluorospot Assay Automation Market also faces hurdles related to strict regulatory frameworks and data management requirements. It must ensure compliance with global standards for clinical trials, diagnostics, and therapeutic development, which often vary across regions. Lack of uniform data formats and assay protocols complicates integration into multinational studies. Variability in reporting standards creates risks of delayed approvals and inconsistent outcomes in regulatory submissions. Institutions face pressure to align assay workflows with evolving compliance guidelines, increasing operational complexity. These challenges highlight the need for harmonization and stronger global frameworks to support automation in immunological research.
Market Opportunities:
Expanding Role in Immunotherapy and Vaccine Development
The Elispot and Fluorospot Assay Automation Market presents strong opportunities through its role in advancing immunotherapy and vaccine pipelines. It enables precise monitoring of immune responses, which is critical for developing next-generation treatments. Pharmaceutical and biotechnology companies increasingly adopt automated platforms to accelerate clinical trials and ensure reliable data. Rising investment in cell-based therapies and personalized vaccines further strengthens demand. Growing global focus on emerging infectious diseases and chronic conditions expands the scope for assay automation. This creates a pathway for broader integration across drug development and clinical research sectors.
Adoption Across Emerging Markets and Academic Research
The Elispot and Fluorospot Assay Automation Market is also positioned to benefit from rising adoption in emerging economies and academic institutions. It supports institutions that are expanding research capabilities and investing in modern laboratory infrastructure. Governments and funding agencies are channeling resources into immunology research, creating new growth avenues. Wider accessibility of automated systems will improve standardization across international studies and foster collaborations. Expanding educational programs and partnerships between universities and industry leaders are driving awareness of automation benefits. These developments open long-term opportunities for deeper market penetration and innovation-driven expansion.
Market Segmentation Analysis:
By Product
The Elispot and Fluorospot Assay Automation Market is segmented into analyzers, software, and accessories. Analyzers dominate due to their ability to deliver high-throughput and standardized results, supporting both clinical and research needs. Software solutions are gaining traction with AI-driven imaging and advanced data management features that improve accuracy. Accessories such as plates and reagents remain essential, ensuring consistency and reliability in assay performance across applications.
- For instance, the Mabtech IRIS 2 can read an entire 96-well ELISpot plate in less than 2 minutes, significantly accelerating data acquisition for researchers.
By Application
Key applications include vaccine development, immunotherapy monitoring, diagnostics, and research studies. Vaccine development holds a leading share, with automated assays providing dependable measurement of immune responses. Immunotherapy monitoring is growing quickly, reflecting demand for precise and reproducible data in personalized medicine. Diagnostic applications continue to expand as healthcare providers require advanced tools to evaluate immune activity. Research studies sustain steady demand, with institutions leveraging automation to support high-quality immunological insights.
By End-Use
End-use segmentation highlights pharmaceutical and biotechnology companies, diagnostic laboratories, and academic research institutes. Pharmaceutical and biotechnology companies account for the largest share, using automated platforms in drug discovery and vaccine pipelines. Diagnostic laboratories deploy automation to reduce manual errors, increase efficiency, and strengthen reliability of results. Academic institutes focus on advancing immunology research and translational medicine by adopting automated systems. It demonstrates wide adoption across industries, underscoring the technology’s importance for both innovation and clinical practice.
- For instance, JPT Peptide Technologies showcased its expertise by achieving the 2nd best score among 35 labs in a 2023 global T-cell ELISpot proficiency panel, confirming its high-quality performance in immune monitoring assays.
Segmentations:
By Product
- Analyzers
- Software
- Accessories
By Application
- Vaccine Development
- Immunotherapy Monitoring
- Diagnostics
- Research Studies
By End-Use
- Pharmaceutical Companies
- Biotechnology Companies
- Diagnostic Laboratories
- Academic Research Institutes
By Region
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
Strong Presence of North America in Driving Market Growth
North America accounted for 42% of the Elispot and Fluorospot Assay Automation Market, making it the leading region. The region benefits from advanced healthcare infrastructure, high research investments, and strong industry-academia collaborations. It also gains momentum from government initiatives that support immunology research and vaccine development. Clinical laboratories and research institutions in the United States and Canada deploy automated platforms to improve efficiency and precision. Integration of AI-driven imaging and digital data management strengthens innovation across laboratories. These drivers reinforce North America’s leadership in the adoption of assay automation technologies.
Rapid Growth of Asia-Pacific Through Expanding Healthcare Investments
Asia-Pacific held 29% of the Elispot and Fluorospot Assay Automation Market, emerging as the fastest-growing regional segment. Rising prevalence of infectious diseases and chronic conditions in China, India, and other nations is expanding demand for advanced diagnostic tools. It gains additional traction from government spending in healthcare and expanding clinical trial activity. Pharmaceutical companies are investing heavily in vaccine and immunotherapy development, creating opportunities for automated assay systems. Research institutions and contract research organizations in the region are also building capacity for high-throughput testing. Strengthened infrastructure and rising investments position Asia-Pacific as a critical growth engine.
Steady Expansion Across Europe with Emphasis on Research Excellence
Europe captured 21% of the Elispot and Fluorospot Assay Automation Market, reflecting its established research and diagnostic base. The region focuses strongly on translational medicine, immunological studies, and collaborative vaccine programs. It benefits from regulatory frameworks that encourage adoption of automated platforms for reliable and standardized testing. Leading research consortia and government-funded projects drive deeper integration of assay automation. Hospitals and diagnostic centers are incorporating these systems to improve accuracy and throughput in routine workflows. These efforts ensure Europe maintains steady momentum within the global market landscape.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- Oxford Immunotec
- U-CyTech
- Abcam Limited
- Bio-Techne Corporation
- BD
- Lophius Biosciences GmbH
- Cellular Technology Limited
- Bio-Connect B.V.
- Autoimmun Diagnostika GmbH
- Mabtech
Competitive Analysis:
The Elispot and Fluorospot Assay Automation Market features a competitive landscape shaped by established technology providers and specialized assay developers. Companies focus on delivering advanced analyzers, digital imaging platforms, and integrated software to enhance sensitivity, throughput, and reproducibility. It is characterized by strong investment in research and development, with firms pursuing innovations in multiplexing and AI-driven data analysis. Strategic collaborations between industry players, academic institutions, and contract research organizations support product validation and global adoption. Market participants also prioritize expanding their distribution networks to reach emerging economies, where healthcare infrastructure is advancing rapidly. Competition is defined by continuous product differentiation, regulatory compliance, and strong after-sales service, ensuring customer confidence in automated platforms. This environment highlights a balance between innovation and scalability, positioning automation technologies as indispensable tools for clinical diagnostics, immunology research, and therapeutic development across global markets.
Recent Developments:
- In January 2025, BD partnered with Biosero to integrate robotic systems with its flow cytometry instruments, aiming to enhance automation in drug discovery and development.
- In April 2025, Mabtech, in partnership with Qamcom, launched a new multiplex platform named EYRA, an image-based system designed for immunology research.
Report Coverage:
The research report offers an in-depth analysis based on Product, Application, End-Use and Region. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook:
- The Elispot and Fluorospot Assay Automation Market will expand with increasing demand for high-throughput immune monitoring tools.
- It will gain momentum from rising adoption in vaccine pipelines and immunotherapy development programs worldwide.
- Automation will strengthen its role by reducing manual errors, improving reproducibility, and ensuring standardized workflows.
- Integration of AI-driven imaging and digital platforms will enhance data accuracy and enable multiparametric immune analysis.
- Pharmaceutical and biotechnology companies will continue to drive adoption to accelerate drug discovery and regulatory approvals.
- Diagnostic laboratories will integrate automated systems to manage higher test volumes and deliver reliable patient outcomes.
- Academic and research institutes will adopt automation to advance translational medicine and personalized healthcare studies.
- Emerging economies will present growth opportunities supported by expanding healthcare infrastructure and clinical research initiatives.
- Strategic collaborations between technology providers and research institutions will shape innovation and expand global reach.
- Future market development will focus on improving accessibility, scalability, and interoperability of automated assay platforms.




