Market Overview:
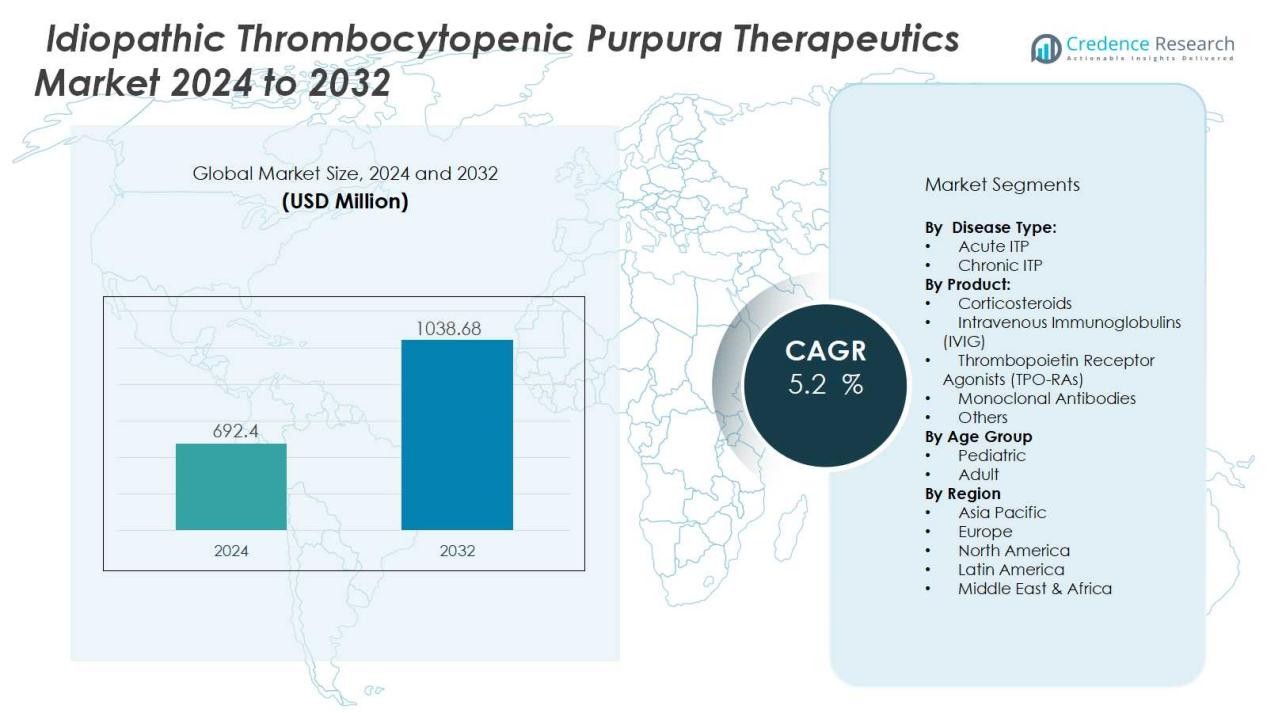
The Idiopathic Thrombocytopenic Purpura Therapeutics Market size was valued at USD 692.4 million in 2024 and is anticipated to reach USD 1038.68 million by 2032, at a CAGR of 5.2 % during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Idiopathic Thrombocytopenic Purpura Therapeutics Market Size 2024 |
USD 692.4 Million |
| Idiopathic Thrombocytopenic Purpura Therapeutics Market, CAGR |
5.2 % |
| Idiopathic Thrombocytopenic Purpura Therapeutics Market Size 2032 |
USD 1038.68 Million |
Key drivers of the market include the rising prevalence of ITP across both pediatric and adult populations, coupled with growing adoption of targeted therapies. Advancements in biologics and monoclonal antibodies have significantly enhanced treatment effectiveness and patient outcomes. Expanding regulatory approvals for new drugs, coupled with favorable reimbursement frameworks in developed markets, further strengthen market expansion. The demand for personalized treatment approaches also drives innovation and competition among biopharmaceutical players.
Regionally, North America dominates the Idiopathic Thrombocytopenic Purpura Therapeutics Market, owing to advanced healthcare infrastructure, strong presence of pharmaceutical companies, and high treatment adoption rates. Europe follows closely with a strong research base and supportive government healthcare policies. Asia-Pacific is expected to register the fastest growth due to increasing awareness, improving healthcare access, and rising investments in rare disease management, making it a key region for future expansion.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights:
- The Idiopathic Thrombocytopenic Purpura Therapeutics Market was valued at USD 692.4 million in 2024 and is expected to reach USD 1038.68 million by 2032, growing at a CAGR of 5.2%.
- Rising prevalence of ITP across pediatric and adult populations continues to drive steady therapeutic demand.
- Biologic therapies and monoclonal antibodies are gaining strong adoption due to higher efficacy and safety.
- Expanding regulatory approvals and active clinical research pipelines strengthen innovation and market competitiveness.
- High treatment costs and limited reimbursement policies in developing regions remain key barriers to growth.
- North America leads with 42% share, supported by advanced infrastructure, strong pharma presence, and favorable reimbursement.
- Asia-Pacific holds 22% share and is the fastest-growing region, driven by rising awareness, healthcare access, and rare disease programs.
Market Drivers:
Rising Prevalence of Idiopathic Thrombocytopenic Purpura Across Age Groups:
The increasing incidence of ITP in both adults and children continues to drive therapeutic demand. Growing awareness and early diagnosis are leading to higher treatment initiation rates. It supports steady adoption of both first-line corticosteroids and second-line biologics. The Idiopathic Thrombocytopenic Purpura Therapeutics Market benefits from this expanding patient base, creating consistent growth opportunities.
- For instance, following the August 2025 FDA approval of Sanofi’s Wayrilz, the company noted that more than 25,000 adults in the U.S. with immune thrombocytopenia could benefit from this advanced treatment.
Advancements in Biologic Therapies and Monoclonal Antibodies:
The shift toward targeted therapies has significantly improved treatment outcomes. Biologic drugs and monoclonal antibodies offer more precise mechanisms of action with better efficacy. Their ability to reduce platelet destruction has made them vital in treatment pathways. It encourages both established and emerging players to focus on advanced product development and portfolio expansion.
Supportive Regulatory Approvals and Expanding Research Pipelines:
Increasing regulatory approvals for novel therapies provide strong momentum for market growth. Companies are investing in clinical trials to expand treatment indications and improve efficacy profiles. Robust pipelines of investigational drugs highlight the industry’s commitment to innovation. It helps accelerate patient access to advanced options while fostering greater competition in the marketplace.
- For instance, the August 2025 FDA approval of Sanofi’s Wayrilz (rilzabrutinib) was based on the LUNA 3 clinical study, in which 31 adult patients achieved a durable platelet response, compared to zero patients in the placebo group.
Growing Healthcare Access and Favorable Reimbursement Policies:
Expanding healthcare coverage in developed markets strengthens adoption of high-cost biologics. Supportive reimbursement frameworks make advanced treatments more accessible to patients. Rising investments in rare disease management enhance global treatment availability. The Idiopathic Thrombocytopenic Purpura Therapeutics Market gains further traction in emerging economies with improving healthcare infrastructure and awareness initiatives.
Market Trends:
Adoption of Targeted and Personalized Therapies in Treatment Pathways:
The market is witnessing a strong shift toward targeted therapies and personalized medicine. Physicians are increasingly prescribing biologics and monoclonal antibodies to address treatment-resistant ITP cases. Advances in genetic and molecular profiling support individualized approaches that improve patient outcomes. Pharmaceutical companies are investing heavily in R&D to expand their biologic pipelines. It creates opportunities for new entrants while pushing established firms to differentiate their therapies. The Idiopathic Thrombocytopenic Purpura Therapeutics Market reflects this trend through higher adoption of advanced treatments across major regions.
- For instance, in August 2025, Sanofi received FDA approval for Wayrilz (rilzabrutinib), making it the first Bruton’s tyrosine kinase (BTK) inhibitor approved for adult ITP patients who had an insufficient response to prior therapy.
Integration of Digital Tools and Expansion of Global Clinical Trials:
The use of digital health tools is enhancing patient monitoring and disease management. Wearable devices and telemedicine platforms allow real-time tracking of platelet levels and treatment adherence. Global pharmaceutical firms are expanding clinical trials to include emerging markets with larger patient populations. This strategy improves access to innovative therapies while diversifying trial data. It also increases collaboration between research institutions and healthcare providers. Growing partnerships are helping accelerate approval timelines and strengthen therapeutic portfolios. The Idiopathic Thrombocytopenic Purpura Therapeutics Market is evolving rapidly with these technology-driven and research-focused strategies.
- For instance, HUTCHMED completed the enrollment of 188 adult patients in China for its pivotal Phase III ESLIM-01 trial of sovleplenib for primary immune thrombocytopenia in December 2022.
Market Challenges Analysis:
High Treatment Costs and Limited Accessibility in Emerging Regions:
The cost of biologics and advanced therapies remains a significant barrier for patients in low- and middle-income countries. Limited healthcare budgets and lack of reimbursement policies restrict access to effective treatments. Many patients rely on corticosteroids as the primary option despite their long-term side effects. It reduces the overall adoption of innovative therapies in these regions. The Idiopathic Thrombocytopenic Purpura Therapeutics Market faces slower growth where affordability issues persist. Addressing this challenge requires strategic pricing models and stronger healthcare funding initiatives.
Adverse Effects, Relapse Risks, and Regulatory Hurdles:
Current treatment options often involve side effects that impact patient compliance and satisfaction. Relapse remains a common issue, forcing repeated treatment cycles and increasing overall costs. Drug development is slowed by stringent regulatory frameworks and extended clinical trial timelines. It delays patient access to potentially life-saving therapies. Competition among companies is also affected, limiting the speed of innovation. The Idiopathic Thrombocytopenic Purpura Therapeutics Market must navigate these hurdles to ensure sustained adoption and long-term success.
Market Opportunities:
Expansion of Biologic and Novel Therapeutic Pipelines:
The growing demand for advanced treatment options creates opportunities for pharmaceutical and biotech firms. Biologics, monoclonal antibodies, and next-generation therapies are gaining strong interest from healthcare providers. Companies are expanding their research pipelines to introduce more effective and safer drugs. Strategic collaborations between global players and regional firms are enhancing innovation and market reach. It positions the Idiopathic Thrombocytopenic Purpura Therapeutics Market for stronger growth through product diversification. Early-stage trials and targeted therapies provide pathways for addressing unmet medical needs.
Rising Focus on Emerging Markets and Digital Healthcare Integration:
Developing regions offer significant growth potential due to increasing awareness and improving healthcare infrastructure. Rising investments in rare disease management programs strengthen access to advanced treatments in Asia-Pacific, Latin America, and the Middle East. Telemedicine platforms and digital monitoring tools provide opportunities to improve treatment adherence and patient outcomes. Pharmaceutical companies are leveraging digital solutions to strengthen engagement with patients and physicians. It enables better disease tracking and customized treatment strategies. The Idiopathic Thrombocytopenic Purpura Therapeutics Market benefits from these trends, creating long-term opportunities for sustainable expansion.
Market Segmentation Analysis:
By Disease Type:
The Idiopathic Thrombocytopenic Purpura Therapeutics Market is segmented into acute and chronic forms. Acute ITP dominates among children, often requiring short-term therapies such as corticosteroids. Chronic ITP is more common in adults, driving long-term demand for biologics and monoclonal antibodies. It benefits from continuous treatment adoption due to relapse risks. Growing diagnosis rates in both categories support segment expansion.
- For instance, a single course of high-dose dexamethasone in adults with newly diagnosed ITP led to a complete response in 27 out of 30 patients within the first seven days.
By Product:
The market includes corticosteroids, intravenous immunoglobulins (IVIG), thrombopoietin receptor agonists (TPO-RAs), monoclonal antibodies, and others. Corticosteroids remain the first-line treatment but face limitations from side effects. TPO-RAs and monoclonal antibodies are witnessing rising adoption due to improved efficacy and better safety profiles. It drives innovation as companies expand their biologic portfolios. IVIG therapies maintain steady demand in emergency and pediatric cases, strengthening product diversity.
- For instance, in a phase 3 study of efgartigimod for chronic ITP, patients achieved a sustained platelet count of 50,000 or more (per µL) for at least 4 of the last 6 weeks, demonstrating reliable platelet improvement.
By Age Group:
Segmentation by age group highlights pediatric and adult populations. Pediatric cases are largely acute and often resolve within months, but they still create demand for supportive therapies. Adult cases are more likely to progress into chronic conditions, driving sustained treatment needs. It emphasizes the growing role of advanced biologics and targeted therapies in adult management. Rising awareness across both groups continues to support stronger adoption rates.
Segmentations:
By Disease Type:
By Product:
- Corticosteroids
- Intravenous Immunoglobulins (IVIG)
- Thrombopoietin Receptor Agonists (TPO-RAs)
- Monoclonal Antibodies
- Others
By Age Group:
By Region:
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
North America:
North America holds 42% market share in the Idiopathic Thrombocytopenic Purpura Therapeutics Market, supported by high treatment adoption and advanced healthcare facilities. The region benefits from the strong presence of pharmaceutical leaders and a favorable reimbursement structure. Rising prevalence of autoimmune disorders has accelerated the use of biologics and monoclonal antibodies. It continues to drive innovation through heavy R&D investments and clinical trials. Patient awareness and early diagnosis rates are high, strengthening demand for advanced therapies. Regulatory support further ensures rapid access to newly approved drugs, reinforcing regional dominance.
Europe:
Europe accounts for 28% market share, driven by robust healthcare systems and a strong research base. Government-funded rare disease programs improve access to therapies and encourage clinical studies. It benefits from collaborations between research institutes and pharmaceutical companies, strengthening treatment pipelines. Increasing regulatory approvals for biologics and wider adoption of targeted therapies support growth. Patient advocacy groups across major countries are playing a critical role in raising awareness. Reimbursement policies across leading European markets ensure patient access to high-cost treatments.
Asia-Pacific:
Asia-Pacific captures 22% market share and is the fastest-growing regional segment, supported by rising healthcare investments and improving access. Growing awareness of rare diseases and expanding diagnostic capabilities are increasing treatment adoption. It attracts pharmaceutical firms that seek opportunities in large patient populations. Governments in key countries are allocating funds for rare disease management programs. Rapid urbanization and healthcare infrastructure development further strengthen regional growth. International collaborations and clinical trials are positioning Asia-Pacific as a key contributor to future market expansion.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
Competitive Analysis:
The Idiopathic Thrombocytopenic Purpura Therapeutics Market is highly competitive, with key players focusing on innovation and strategic growth. Leading companies such as Amgen Inc., F. Hoffmann-La Roche Ltd, Grifols, S.A., and GSK plc. dominate through strong product portfolios and continuous investments in biologics. Medtronic, Shangxian Minimal Invassive Inc., and INTROMEDIC are also enhancing their presence by expanding therapeutic solutions and strengthening distribution networks. It is characterized by active research pipelines and rising adoption of targeted therapies. Companies are prioritizing regulatory approvals and clinical trial expansions to strengthen their competitive positioning. Partnerships, acquisitions, and collaborations are frequently pursued to diversify offerings and increase global reach. Strong competition encourages faster innovation and more cost-effective treatment strategies. The focus on personalized medicine and advanced biologics ensures a dynamic environment where established leaders and emerging players compete to meet evolving patient needs.
Recent Developments:
- In October 2025, Amgen announced the expansion of Repatha (evolocumab) use after positive Phase 3 trial results and secured FDA approval to cover adults at high risk of major cardiovascular events.
- In June 2024, Grifols completed the sale of a 20% stake in Shanghai RAAS and began a strategic alliance with Haier Group, including an exclusive albumin distribution agreement.
Report Coverage:
The research report offers an in-depth analysis based on Disease Type, Product, Age Group and Region. It details leading Market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current Market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven Market expansion in recent years. The report also explores Market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on Market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the Market.
Future Outlook:
- The Idiopathic Thrombocytopenic Purpura Therapeutics Market will witness stronger adoption of biologics and monoclonal antibodies in treatment pathways.
- Targeted therapies will gain preference as physicians prioritize improved safety and efficacy over traditional options.
- Rising investments in clinical trials will expand the drug pipeline and introduce novel treatment options.
- Digital health tools, including remote monitoring platforms, will enhance patient adherence and disease tracking.
- Regulatory approvals for innovative drugs will accelerate patient access and strengthen competitive dynamics.
- Emerging markets in Asia-Pacific and Latin America will play a critical role in driving demand growth.
- Collaborations between global pharmaceutical firms and regional healthcare providers will expand treatment availability.
- Personalized medicine will become more prominent, with genetic profiling guiding therapy selection and patient management.
- Patient advocacy initiatives will strengthen awareness, encouraging earlier diagnosis and treatment adoption.
- The Idiopathic Thrombocytopenic Purpura Therapeutics Market will evolve with continued innovation, expanded accessibility, and improved patient outcomes worldwide.




