CHAPTER NO. 1 : INTRODUCTION 31
1.1.1. Report Description 31
Purpose of the Report 31
USP & Key Offerings 31
1.1.2. Key Benefits for Stakeholders 31
1.1.3. Target Audience 32
1.1.4. Report Scope 32
1.1.5. Regional Scope 33
CHAPTER NO. 2 : EXECUTIVE SUMMARY 34
2.1. Medical Device Contract Manufacturing Market Snapshot 34
2.1.1. Global Medical Device Contract Manufacturing Market, 2018 – 2032 (USD Million) 36
CHAPTER NO. 3 : GEOPOLITICAL CRISIS IMPACT ANALYSIS 37
3.1. Russia-Ukraine and Israel-Palestine War Impacts 37
CHAPTER NO. 4 : MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – INDUSTRY ANALYSIS 38
4.1. Introduction 38
4.2. Market Drivers 39
4.2.1. Aging Population and Increased Healthcare Demand 39
4.2.2. Advancements in Manufacturing Technologies 40
4.3. Market Restraints 41
4.3.1. High Research and Development Costs 41
4.4. Market Opportunities 42
4.4.1. Market Opportunity Analysis 42
4.5. Porter’s Five Forces Analysis 43
CHAPTER NO. 5 : ANALYSIS COMPETITIVE LANDSCAPE 44
5.1. Company Market Share Analysis – 2023 44
5.1.1. Global Medical Device Contract Manufacturing Market: Company Market Share, by Volume, 2023 44
5.1.2. Global Medical Device Contract Manufacturing Market: Company Market Share, by Revenue, 2023 45
5.1.3. Global Medical Device Contract Manufacturing Market: Top 6 Company Market Share, by Revenue, 2023 45
5.1.4. Global Medical Device Contract Manufacturing Market: Top 3 Company Market Share, by Revenue, 2023 46
5.2. Global Medical Device Contract Manufacturing Market Company Revenue Market Share, 2023 47
5.3. Company Assessment Metrics, 2023 48
5.3.1. Stars 48
5.3.2. Emerging Leaders 48
5.3.3. Pervasive Players 48
5.3.4. Participants 48
5.4. Start-ups /SMEs Assessment Metrics, 2023 48
5.4.1. Progressive Companies 48
5.4.2. Responsive Companies 48
5.4.3. Dynamic Companies 48
5.4.4. Starting Blocks 48
5.5. Strategic Developments 49
5.5.1. Acquisitions & Mergers 49
New Product Launch 49
Regional Expansion 49
5.6. Key Players Product Matrix 50
CHAPTER NO. 6 : PESTEL & ADJACENT MARKET ANALYSIS 51
6.1. PESTEL 51
6.1.1. Political Factors 51
6.1.2. Economic Factors 51
6.1.3. Social Factors 51
6.1.4. Technological Factors 51
6.1.5. Environmental Factors 51
6.1.6. Legal Factors 51
6.2. Adjacent Market Analysis 51
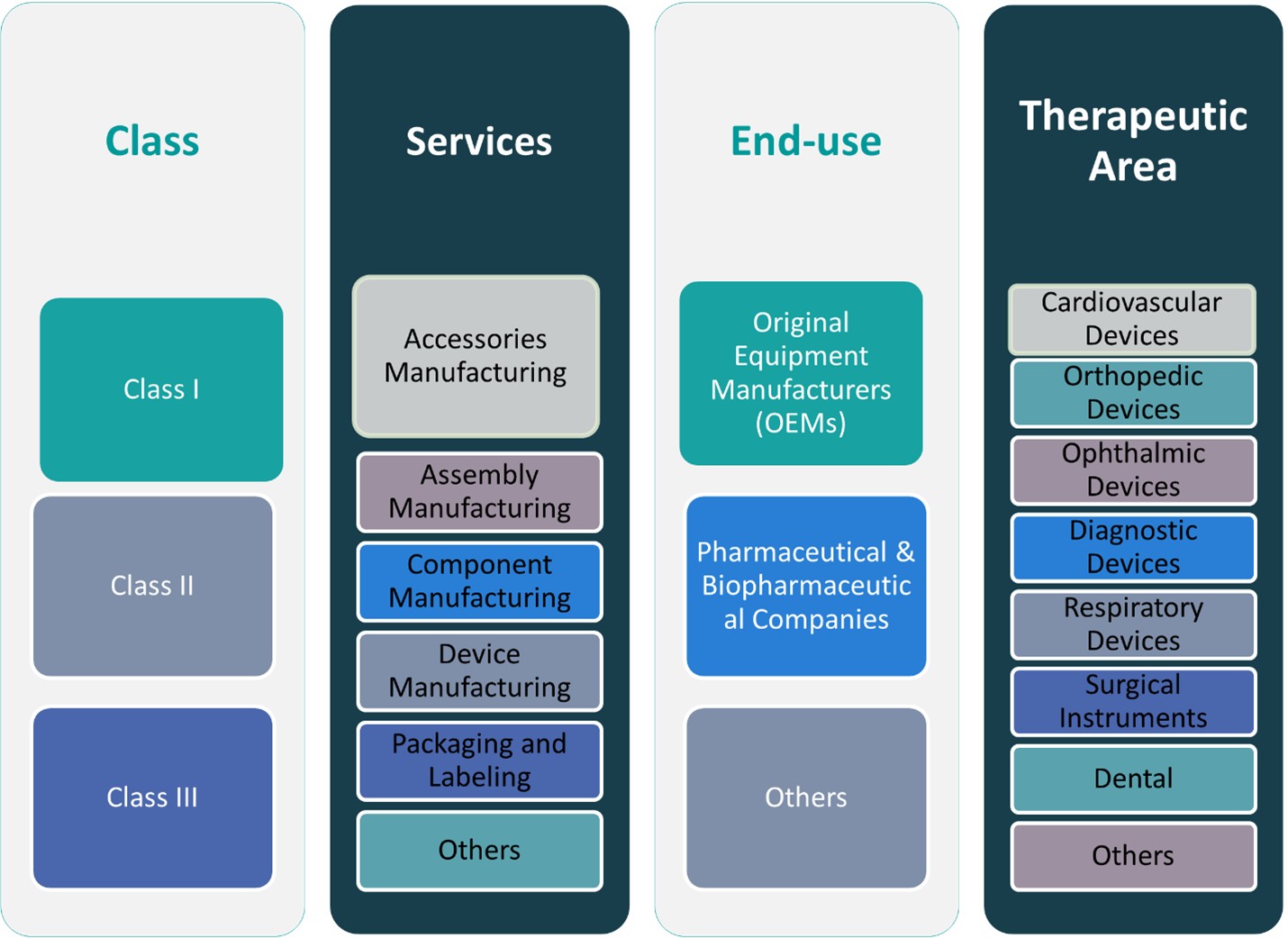
CHAPTER NO. 7 : MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – BY CLASS SEGMENT ANALYSIS 52
7.1. Medical Device Contract Manufacturing Market Overview, by Class Segment 52
7.1.1. Medical Device Contract Manufacturing Market Revenue Share, By Class, 2023 & 2032 53
7.1.2. Medical Device Contract Manufacturing Market Attractiveness Analysis, By Class 54
7.1.3. Incremental Revenue Growth Opportunity, by Class, 2024 – 2032 54
7.1.4. Medical Device Contract Manufacturing Market Revenue, By Class, 2018, 2023, 2027 & 2032 55
7.2. Class I 56
7.2.1. Global Class I Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 57
7.2.2. Global Class I Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 57
7.3. Class II 58
7.3.1. Global Class II Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 59
7.3.2. Global Class II Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 59
7.4. Class III 60
7.4.1. Global Class III Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 61
7.4.2. Global Class III Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 61
CHAPTER NO. 8 : MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – BY SERVICES SEGMENT ANALYSIS 62
8.1. Medical Device Contract Manufacturing Market Overview, by Services Segment 62
8.1.1. Medical Device Contract Manufacturing Market Revenue Share, By Services, 2023 & 2032 63
8.1.2. Medical Device Contract Manufacturing Market Attractiveness Analysis, By Services 64
8.1.3. Incremental Revenue Growth Opportunity, by Services, 2024 – 2032 64
8.1.4. Medical Device Contract Manufacturing Market Revenue, By Services, 2018, 2023, 2027 & 2032 65
8.2. Accessories Manufacturing 66
8.2.1. Global Accessories Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 67
8.2.2. Global Accessories Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 67
8.3. Assembly Manufacturing 68
8.3.1. Global Assembly Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 69
8.3.2. Global Assembly Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 69
8.4. Component Manufacturing 70
8.4.1. Global Component Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 71
8.4.2. Global Component Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 71
8.5. Device Manufacturing 72
8.5.1. Global Device Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 73
8.5.2. Global Device Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 73
8.6. Packaging and Labelling 74
8.6.1. Global Packaging and Labeling Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 75
8.6.2. Global Packaging and Labeling Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 75
8.7. Others 76
8.7.1. Global Others Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 77
8.7.2. Global Others Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 77
CHAPTER NO. 9 : MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – BY END-USE SEGMENT ANALYSIS 78
9.1. Medical Device Contract Manufacturing Market Overview, by End-use Segment 78
9.1.1. Medical Device Contract Manufacturing Market Revenue Share, By End-user, 2023 & 2032 79
9.1.2. Medical Device Contract Manufacturing Market Attractiveness Analysis, By End-user 80
9.1.3. Incremental Revenue Growth Opportunity, by End-user, 2024 – 2032 80
9.1.4. Medical Device Contract Manufacturing Market Revenue, By End-user, 2018, 2023, 2027 & 2032 81
9.2. Original Equipment Manufacturers (OEMs) 82
9.2.1. Global Original Equipment Manufacturers (OEMs) Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 83
9.2.2. Global Original Equipment Manufacturers (OEMs) Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 83
9.3. Pharmaceutical & Biopharmaceutical Companies 84
9.3.1. Global Pharmaceutical & Biopharmaceutical Companies Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 85
9.3.2. Global Pharmaceutical & Biopharmaceutical Companies Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 85
9.4. Others 86
9.4.1. Global Others Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 87
9.4.2. Global Others Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 87
CHAPTER NO. 10 : MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – BY THERAPEUTIC AREA SEGMENT ANALYSIS 88
10.1. Medical Device Contract Manufacturing Market Overview, by Therapeutic Area Segment 88
10.1.1. Medical Device Contract Manufacturing Market Revenue Share, By Therapeutic Area, 2023 & 2032 89
10.1.2. Medical Device Contract Manufacturing Market Attractiveness Analysis, By Therapeutic Area 90
10.1.3. Incremental Revenue Growth Opportunity, by Therapeutic Area, 2024 – 2032 90
10.1.4. Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018, 2023, 2027 & 2032 91
10.2. Cardiovascular Devices 92
10.2.1. Global Cardiovascular Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 93
10.2.2. Global Cardiovascular Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 93
10.3. Orthopedic Devices 94
10.3.1. Global Orthopedic Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 95
10.3.2. Global Orthopedic Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 95
10.4. Ophthalmic Devices 96
10.4.1. Global Ophthalmic Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 97
10.4.2. Global Ophthalmic Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 97
10.5. Diagnostic Devices 98
10.5.1. Global Diagnostic Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 99
10.5.2. Global Diagnostic Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 99
10.6. Respiratory Devices 100
10.6.1. Global Respiratory Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 101
10.6.2. Global Respiratory Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 101
10.7. Surgical Instruments 102
10.7.1. Global Surgical Instruments Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 103
10.7.2. Global Surgical Instruments Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 103
10.8. Dental 104
10.8.1. Global Dental Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 105
10.8.2. Global Dental Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 105
10.9. Others 106
10.9.1. Global Others Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 107
10.9.2. Global Others Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 107
CHAPTER NO. 11 : MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – REGIONAL ANALYSIS 108
11.1. Medical Device Contract Manufacturing Market Overview, by Regional Segments 108
11.2. Region 109
11.2.1. Global Medical Device Contract Manufacturing Market Revenue Share, By Region, 2023 & 2032 109
11.2.2. Medical Device Contract Manufacturing Market Attractiveness Analysis, By Region 110
11.2.3. Incremental Revenue Growth Opportunity, by Region, 2024 – 2032 110
11.2.4. Medical Device Contract Manufacturing Market Revenue, By Region, 2018, 2023, 2027 & 2032 111
11.2.5. Global Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 112
11.2.6. Global Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 112
11.3. Class 113
11.3.1. Global Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 113
11.3.2. Global Medical Device Contract Manufacturing Market Revenue, By Class, 2024 – 2032 (USD Million) 113
11.4. Services 114
11.4.1. Global Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 114
11.4.2. Global Medical Device Contract Manufacturing Market Revenue, By Services, 2024 – 2032 (USD Million) 114
11.5. End-use 115
11.5.1. Global Medical Device Contract Manufacturing Market Revenue, By End-use, 2018 – 2023 (USD Million) 115
11.5.2. Global Medical Device Contract Manufacturing Market Revenue, By End-use, 2024 – 2032 (USD Million) 115
11.6. Therapeutic Area 116
11.6.1. Global Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 116
11.6.2. Global Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2024 – 2032 (USD Million) 116
CHAPTER NO. 12 : MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – NORTH AMERICA 117
12.1. North America 117
12.1.1. Key Highlights 117
12.1.2. North America Medical Device Contract Manufacturing Market Revenue, By Country, 2018 – 2023 (USD Million) 118
12.1.3. North America Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 119
12.1.4. North America Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 120
12.1.5. North America Medical Device Contract Manufacturing Market Revenue, By End-user, 2018 – 2023 (USD Million) 121
12.1.6. North America Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 122
12.2. U.S. 123
12.3. Canada 123
12.4. Mexico 123
CHAPTER NO. 13 : MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – EUROPE 124
13.1. Europe 124
13.1.1. Key Highlights 124
13.1.2. Europe Medical Device Contract Manufacturing Market Revenue, By Country, 2018 – 2023 (USD Million) 125
13.1.3. Europe Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 126
13.1.4. Europe Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 127
13.1.5. Europe Medical Device Contract Manufacturing Market Revenue, By End-user, 2018 – 2023 (USD Million) 128
13.1.6. Europe Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 129
13.2. UK 130
13.3. France 130
13.4. Germany 130
13.5. Italy 130
13.6. Spain 130
13.7. Russia 130
13.8. Belgium 130
13.9. Netherland 130
13.10. Austria 130
13.11. Sweden 130
13.12. Poland 130
13.13. Denmark 130
13.14. Switzerland 130
13.15. Rest of Europe 130
CHAPTER NO. 14 : MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – ASIA PACIFIC 131
14.1. Asia Pacific 131
14.1.1. Key Highlights 131
14.1.2. Asia Pacific Medical Device Contract Manufacturing Market Revenue, By Country, 2018 – 2023 (USD Million) 132
14.1.3. Asia Pacific Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 133
14.1.4. Asia Pacific Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 134
14.1.5. Asia Pacific Medical Device Contract Manufacturing Market Revenue, By End-user, 2018 – 2023 (USD Million) 135
14.1.6. Asia Pacific Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 136
14.2. China 137
14.3. Japan 137
14.4. South Korea 137
14.5. India 137
14.6. Australia 137
14.7. Thailand 137
14.8. Indonesia 137
14.9. Vietnam 137
14.10. Malaysia 137
14.11. Philippines 137
14.12. Taiwan 137
14.13. Rest of Asia Pacific 137
CHAPTER NO. 15 : MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – LATIN AMERICA 138
15.1. Latin America 138
15.1.1. Key Highlights 138
15.1.2. Latin America Medical Device Contract Manufacturing Market Revenue, By Country, 2018 – 2023 (USD Million) 139
15.1.3. Latin America Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 140
15.1.4. Latin America Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 141
15.1.5. Latin America Medical Device Contract Manufacturing Market Revenue, By End-user, 2018 – 2023 (USD Million) 142
15.1.6. Latin America Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 143
15.2. Brazil 144
15.3. Argentina 144
15.4. Peru 144
15.5. Chile 144
15.6. Colombia 144
15.7. Rest of Latin America 144
CHAPTER NO. 16 : MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – MIDDLE EAST 145
16.1. Middle East 145
16.1.1. Key Highlights 145
16.1.2. Middle East Medical Device Contract Manufacturing Market Revenue, By Country, 2018 – 2023 (USD Million) 146
16.1.3. Middle East Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 147
16.1.4. Middle East Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 148
16.1.5. Middle East Medical Device Contract Manufacturing Market Revenue, By End-user, 2018 – 2023 (USD Million) 149
16.1.6. Middle East Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 150
16.2. UAE 151
16.3. KSA 151
16.4. Israel 151
16.5. Turkey 151
16.6. Iran 151
16.7. Rest of Middle East 151
CHAPTER NO. 17 : MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – AFRICA 152
17.1. Africa 152
17.1.1. Key Highlights 152
17.1.2. Africa Medical Device Contract Manufacturing Market Revenue, By Country, 2018 – 2023 (USD Million) 153
17.1.3. Africa Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 154
17.1.4. Africa Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 155
17.1.5. Africa Medical Device Contract Manufacturing Market Revenue, By End-user, 2018 – 2023 (USD Million) 156
17.1.6. Africa Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 157
17.2. Egypt 158
17.3. Nigeria 158
17.4. Algeria 158
17.5. Morocco 158
17.6. Rest of Africa 158
CHAPTER NO. 18 : COMPANY PROFILES 159
18.1. Jabil Inc. 159
18.1.1. Company Overview 159
18.1.2. Product Portfolio 159
18.1.3. Swot Analysis 159
18.1.4. Business Strategy 160
18.1.5. Financial Overview 160
18.2. Thermo Fisher Scientific Inc. 161
18.3. Integer Holdings Corporation 161
18.4. Sanmina Corporation 161
18.5. Celestica Inc. 161
18.6. Company 6 161
18.7. Company 7 161
18.8. Company 8 161
18.9. Company 9 161
18.10. Company 10 161
18.11. Company 11 161
18.12. Company 12 161
18.13. Company 13 161
18.14. Company 14 161
List of Figures
FIG NO. 1. Global Medical Device Contract Manufacturing Market Revenue, 2018 – 2032 (USD Million) 36
FIG NO. 2. Porter’s Five Forces Analysis for Global Medical Device Contract Manufacturing Market 43
FIG NO. 3. Company Share Analysis, 2023 44
FIG NO. 4. Company Share Analysis, 2023 45
FIG NO. 5. Company Share Analysis, 2023 45
FIG NO. 6. Company Share Analysis, 2023 46
FIG NO. 7. Medical Device Contract Manufacturing Market – Company Revenue Market Share, 2023 47
FIG NO. 8. Medical Device Contract Manufacturing Market Revenue Share, By Class, 2023 & 2032 53
FIG NO. 9. Market Attractiveness Analysis, By Class 54
FIG NO. 10. Incremental Revenue Growth Opportunity by Class, 2024 – 2032 54
FIG NO. 11. Medical Device Contract Manufacturing Market Revenue, By Class, 2018, 2023, 2027 & 2032 55
FIG NO. 12. Global Medical Device Contract Manufacturing Market for Class I, Revenue (USD Million) 2018 – 2032 56
FIG NO. 13. Global Medical Device Contract Manufacturing Market for Class II, Revenue (USD Million) 2018 – 2032 58
FIG NO. 14. Global Medical Device Contract Manufacturing Market for Class III, Revenue (USD Million) 2018 – 2032 60
FIG NO. 15. Medical Device Contract Manufacturing Market Revenue Share, By Services, 2023 & 2032 63
FIG NO. 16. Market Attractiveness Analysis, By Services 64
FIG NO. 17. Incremental Revenue Growth Opportunity by Services, 2024 – 2032 64
FIG NO. 18. Medical Device Contract Manufacturing Market Revenue, By Services, 2018, 2023, 2027 & 2032 65
FIG NO. 19. Global Medical Device Contract Manufacturing Market for Accessories Manufacturing, Revenue (USD Million) 2018 – 2032 66
FIG NO. 20. Global Medical Device Contract Manufacturing Market for Assembly Manufacturing, Revenue (USD Million) 2018 – 2032 68
FIG NO. 21. Global Medical Device Contract Manufacturing Market for Component Manufacturing, Revenue (USD Million) 2018 – 2032 70
FIG NO. 22. Global Medical Device Contract Manufacturing Market for Device Manufacturing, Revenue (USD Million) 2018 – 2032 72
FIG NO. 23. Global Medical Device Contract Manufacturing Market for Packaging and Labeling, Revenue (USD Million) 2018 – 2032 74
FIG NO. 24. Global Medical Device Contract Manufacturing Market for Others, Revenue (USD Million) 2018 – 2032 76
FIG NO. 25. Medical Device Contract Manufacturing Market Revenue Share, By End-user, 2023 & 2032 79
FIG NO. 26. Market Attractiveness Analysis, By End-user 80
FIG NO. 27. Incremental Revenue Growth Opportunity by End-user, 2024 – 2032 80
FIG NO. 28. Medical Device Contract Manufacturing Market Revenue, By End-user, 2018, 2023, 2027 & 2032 81
FIG NO. 29. Global Medical Device Contract Manufacturing Market for Original Equipment Manufacturers (OEMs), Revenue (USD Million) 2018 – 2032 82
FIG NO. 30. Global Medical Device Contract Manufacturing Market for Pharmaceutical & Biopharmaceutical Companies, Revenue (USD Million) 2018 – 2032 84
FIG NO. 31. Global Medical Device Contract Manufacturing Market for Others, Revenue (USD Million) 2018 – 2032 86
FIG NO. 32. Medical Device Contract Manufacturing Market Revenue Share, By Therapeutic Area, 2023 & 2032 89
FIG NO. 33. Market Attractiveness Analysis, By Therapeutic Area 90
FIG NO. 34. Incremental Revenue Growth Opportunity by Therapeutic Area, 2024 – 2032 90
FIG NO. 35. Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018, 2023, 2027 & 2032 91
FIG NO. 36. Global Medical Device Contract Manufacturing Market for Cardiovascular Devices, Revenue (USD Million) 2018 – 2032 92
FIG NO. 37. Global Medical Device Contract Manufacturing Market for Orthopedic Devices, Revenue (USD Million) 2018 – 2032 94
FIG NO. 38. Global Medical Device Contract Manufacturing Market for Ophthalmic Devices, Revenue (USD Million) 2018 – 2032 96
FIG NO. 39. Global Medical Device Contract Manufacturing Market for Diagnostic Devices, Revenue (USD Million) 2018 – 2032 98
FIG NO. 40. Global Medical Device Contract Manufacturing Market for Respiratory Devices, Revenue (USD Million) 2018 – 2032 100
FIG NO. 41. Global Medical Device Contract Manufacturing Market for Surgical Instruments, Revenue (USD Million) 2018 – 2032 102
FIG NO. 42. Global Medical Device Contract Manufacturing Market for Dental, Revenue (USD Million) 2018 – 2032 104
FIG NO. 43. Global Medical Device Contract Manufacturing Market for Others, Revenue (USD Million) 2018 – 2032 106
FIG NO. 44. Global Medical Device Contract Manufacturing Market Revenue Share, By Region, 2023 & 2032 109
FIG NO. 45. Market Attractiveness Analysis, By Region 110
FIG NO. 46. Incremental Revenue Growth Opportunity by Region, 2024 – 2032 110
FIG NO. 47. Medical Device Contract Manufacturing Market Revenue, By Region, 2018, 2023, 2027 & 2032 111
FIG NO. 48. North America Medical Device Contract Manufacturing Market Revenue, 2018 – 2032 (USD Million) 117
FIG NO. 49. Europe Medical Device Contract Manufacturing Market Revenue, 2018 – 2032 (USD Million) 124
FIG NO. 50. Asia Pacific Medical Device Contract Manufacturing Market Revenue, 2018 – 2032 (USD Million) 131
FIG NO. 51. Latin America Medical Device Contract Manufacturing Market Revenue, 2018 – 2032 (USD Million) 138
FIG NO. 52. Middle East Medical Device Contract Manufacturing Market Revenue, 2018 – 2032 (USD Million) 145
FIG NO. 53. Africa Medical Device Contract Manufacturing Market Revenue, 2018 – 2032 (USD Million) 152
List of Tables
TABLE NO. 1. : Global Medical Device Contract Manufacturing Market: Snapshot 34
TABLE NO. 2. : Drivers for the Medical Device Contract Manufacturing Market: Impact Analysis 39
TABLE NO. 3. : Restraints for the Medical Device Contract Manufacturing Market: Impact Analysis 41
TABLE NO. 4. : Global Class I Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 57
TABLE NO. 5. : Global Class I Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 57
TABLE NO. 6. : Global Class II Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 59
TABLE NO. 7. : Global Class II Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 59
TABLE NO. 8. : Global Class III Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 61
TABLE NO. 9. : Global Class III Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 61
TABLE NO. 10. : Global Accessories Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 67
TABLE NO. 11. : Global Accessories Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 67
TABLE NO. 12. : Global Assembly Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 69
TABLE NO. 13. : Global Assembly Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 69
TABLE NO. 14. : Global Component Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 71
TABLE NO. 15. : Global Component Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 71
TABLE NO. 16. : Global Device Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 73
TABLE NO. 17. : Global Device Manufacturing Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 73
TABLE NO. 18. : Global Packaging and Labeling Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 75
TABLE NO. 19. : Global Packaging and Labeling Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 75
TABLE NO. 20. : Global Others Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 77
TABLE NO. 21. : Global Others Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 77
TABLE NO. 22. : Global Original Equipment Manufacturers (OEMs) Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 83
TABLE NO. 23. : Global Original Equipment Manufacturers (OEMs) Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 83
TABLE NO. 24. : Global Pharmaceutical & Biopharmaceutical Companies Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 85
TABLE NO. 25. : Global Pharmaceutical & Biopharmaceutical Companies Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 85
TABLE NO. 26. : Global Others Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 87
TABLE NO. 27. : Global Others Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 87
TABLE NO. 28. : Global Cardiovascular Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 93
TABLE NO. 29. : Global Cardiovascular Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 93
TABLE NO. 30. : Global Orthopedic Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 95
TABLE NO. 31. : Global Orthopedic Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 95
TABLE NO. 32. : Global Ophthalmic Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 97
TABLE NO. 33. : Global Ophthalmic Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 97
TABLE NO. 34. : Global Diagnostic Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 99
TABLE NO. 35. : Global Diagnostic Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 99
TABLE NO. 36. : Global Respiratory Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 101
TABLE NO. 37. : Global Respiratory Devices Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 101
TABLE NO. 38. : Global Surgical Instruments Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 103
TABLE NO. 39. : Global Surgical Instruments Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 103
TABLE NO. 40. : Global Dental Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 105
TABLE NO. 41. : Global Dental Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 105
TABLE NO. 42. : Global Others Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 107
TABLE NO. 43. : Global Others Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 107
TABLE NO. 44. : Global Medical Device Contract Manufacturing Market Revenue, By Region, 2018 – 2023 (USD Million) 112
TABLE NO. 45. : Global Medical Device Contract Manufacturing Market Revenue, By Region, 2024 – 2032 (USD Million) 112
TABLE NO. 46. : Global Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 113
TABLE NO. 47. : Global Medical Device Contract Manufacturing Market Revenue, By Class, 2024 – 2032 (USD Million) 113
TABLE NO. 48. : Global Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 114
TABLE NO. 49. : Global Medical Device Contract Manufacturing Market Revenue, By Services, 2024 – 2032 (USD Million) 114
TABLE NO. 50. : Global Medical Device Contract Manufacturing Market Revenue, By End-use, 2018 – 2023 (USD Million) 115
TABLE NO. 51. : Global Medical Device Contract Manufacturing Market Revenue, By End-use, 2024 – 2032 (USD Million) 115
TABLE NO. 52. : Global Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 116
TABLE NO. 53. : Global Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2024 – 2032 (USD Million) 116
TABLE NO. 54. : North America Medical Device Contract Manufacturing Market Revenue, By Country, 2018 – 2023 (USD Million) 118
TABLE NO. 55. : North America Medical Device Contract Manufacturing Market Revenue, By Country, 2024 – 2032 (USD Million) 118
TABLE NO. 56. : North America Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 119
TABLE NO. 57. : North America Medical Device Contract Manufacturing Market Revenue, By Class, 2024 – 2032 (USD Million) 119
TABLE NO. 58. : North America Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 120
TABLE NO. 59. : North America Medical Device Contract Manufacturing Market Revenue, By Services, 2024 – 2032 (USD Million) 120
TABLE NO. 60. : North America Medical Device Contract Manufacturing Market Revenue, By End-user, 2018 – 2023 (USD Million) 121
TABLE NO. 61. : North America Medical Device Contract Manufacturing Market Revenue, By End-user, 2024 – 2032 (USD Million) 121
TABLE NO. 62. : North America Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 122
TABLE NO. 63. : North America Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2024 – 2032 (USD Million) 122
TABLE NO. 64. : Europe Medical Device Contract Manufacturing Market Revenue, By Country, 2018 – 2023 (USD Million) 125
TABLE NO. 65. : Europe Medical Device Contract Manufacturing Market Revenue, By Country, 2024 – 2032 (USD Million) 125
TABLE NO. 66. : Europe Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 126
TABLE NO. 67. : Europe Medical Device Contract Manufacturing Market Revenue, By Class, 2024 – 2032 (USD Million) 126
TABLE NO. 68. : Europe Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 127
TABLE NO. 69. : Europe Medical Device Contract Manufacturing Market Revenue, By Services, 2024 – 2032 (USD Million) 127
TABLE NO. 70. : Europe Medical Device Contract Manufacturing Market Revenue, By End-user, 2018 – 2023 (USD Million) 128
TABLE NO. 71. : Europe Medical Device Contract Manufacturing Market Revenue, By End-user, 2024 – 2032 (USD Million) 128
TABLE NO. 72. : Europe Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 129
TABLE NO. 73. : Europe Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2024 – 2032 (USD Million) 129
TABLE NO. 74. : Asia Pacific Medical Device Contract Manufacturing Market Revenue, By Country, 2018 – 2023 (USD Million) 132
TABLE NO. 75. : Asia Pacific Medical Device Contract Manufacturing Market Revenue, By Country, 2024 – 2032 (USD Million) 132
TABLE NO. 76. : Asia Pacific Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 133
TABLE NO. 77. : Asia Pacific Medical Device Contract Manufacturing Market Revenue, By Class, 2024 – 2032 (USD Million) 133
TABLE NO. 78. : Asia Pacific Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 134
TABLE NO. 79. : Asia Pacific Medical Device Contract Manufacturing Market Revenue, By Services, 2024 – 2032 (USD Million) 134
TABLE NO. 80. : Asia Pacific Medical Device Contract Manufacturing Market Revenue, By End-user, 2018 – 2023 (USD Million) 135
TABLE NO. 81. : Asia Pacific Medical Device Contract Manufacturing Market Revenue, By End-user, 2024 – 2032 (USD Million) 135
TABLE NO. 82. : Asia Pacific Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 136
TABLE NO. 83. : Asia Pacific Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2024 – 2032 (USD Million) 136
TABLE NO. 84. : Latin America Medical Device Contract Manufacturing Market Revenue, By Country, 2018 – 2023 (USD Million) 139
TABLE NO. 85. : Latin America Medical Device Contract Manufacturing Market Revenue, By Country, 2024 – 2032 (USD Million) 139
TABLE NO. 86. : Latin America Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 140
TABLE NO. 87. : Latin America Medical Device Contract Manufacturing Market Revenue, By Class, 2024 – 2032 (USD Million) 140
TABLE NO. 88. : Latin America Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 141
TABLE NO. 89. : Latin America Medical Device Contract Manufacturing Market Revenue, By Services, 2024 – 2032 (USD Million) 141
TABLE NO. 90. : Latin America Medical Device Contract Manufacturing Market Revenue, By End-user, 2018 – 2023 (USD Million) 142
TABLE NO. 91. : Latin America Medical Device Contract Manufacturing Market Revenue, By End-user, 2024 – 2032 (USD Million) 142
TABLE NO. 92. : Latin America Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 143
TABLE NO. 93. : Latin America Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2024 – 2032 (USD Million) 143
TABLE NO. 94. : Middle East Medical Device Contract Manufacturing Market Revenue, By Country, 2018 – 2023 (USD Million) 146
TABLE NO. 95. : Middle East Medical Device Contract Manufacturing Market Revenue, By Country, 2024 – 2032 (USD Million) 146
TABLE NO. 96. : Middle East Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 147
TABLE NO. 97. : Middle East Medical Device Contract Manufacturing Market Revenue, By Class, 2024 – 2032 (USD Million) 147
TABLE NO. 98. : Middle East Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 148
TABLE NO. 99. : Middle East Medical Device Contract Manufacturing Market Revenue, By Services, 2024 – 2032 (USD Million) 148
TABLE NO. 100. : Middle East Medical Device Contract Manufacturing Market Revenue, By End-user, 2018 – 2023 (USD Million) 149
TABLE NO. 101. : Middle East Medical Device Contract Manufacturing Market Revenue, By End-user, 2024 – 2032 (USD Million) 149
TABLE NO. 102. : Middle East Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 150
TABLE NO. 103. : Middle East Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2024 – 2032 (USD Million) 150
TABLE NO. 104. : Africa Medical Device Contract Manufacturing Market Revenue, By Country, 2018 – 2023 (USD Million) 153
TABLE NO. 105. : Africa Medical Device Contract Manufacturing Market Revenue, By Country, 2024 – 2032 (USD Million) 153
TABLE NO. 106. : Africa Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 154
TABLE NO. 107. : Africa Medical Device Contract Manufacturing Market Revenue, By Class, 2024 – 2032 (USD Million) 154
TABLE NO. 108. : Africa Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 155
TABLE NO. 109. : Africa Medical Device Contract Manufacturing Market Revenue, By Services, 2024 – 2032 (USD Million) 155
TABLE NO. 110. : Africa Medical Device Contract Manufacturing Market Revenue, By End-user, 2018 – 2023 (USD Million) 156
TABLE NO. 111. : Africa Medical Device Contract Manufacturing Market Revenue, By End-user, 2024 – 2032 (USD Million) 156
TABLE NO. 112. : Africa Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 157
TABLE NO. 113. : Africa Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2024 – 2032 (USD Million) 157