| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Japan Electrophysiology Devices Market Size 2024 |
USD 794.06 million |
| Japan Electrophysiology Devices Market, CAGR |
15.24% |
| Japan Electrophysiology Devices Market Size 2032 |
USD 2,470.07 million |
Market Overview
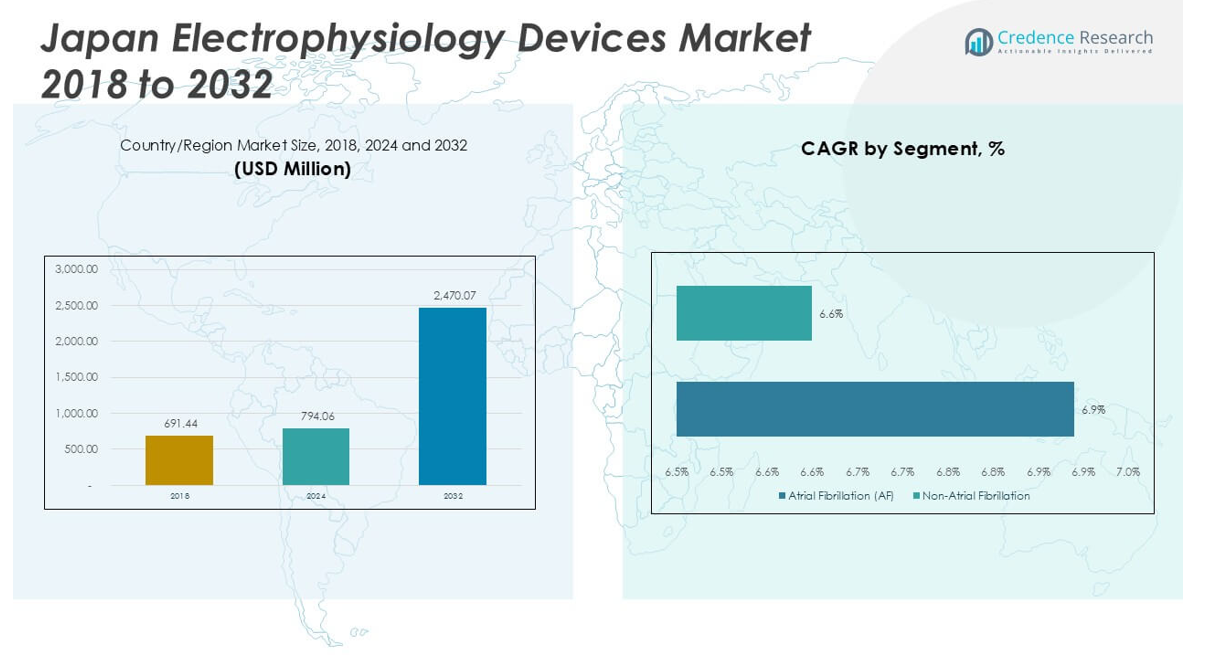
Japan Electrophysiology Devices Market size was valued at USD 691.44 million in 2018 to USD 794.06 million in 2024 and is anticipated to reach USD 2,470.07 million by 2032, at a CAGR of 15.24% during the forecast period.
The Japan Electrophysiology Devices Market experiences strong growth due to the rising prevalence of cardiac arrhythmias, an aging population, and increased adoption of minimally invasive procedures. Advancements in electrophysiology technologies, including enhanced mapping systems and next-generation ablation catheters, drive improved clinical outcomes and procedural efficiency. Supportive government initiatives and favorable reimbursement policies further accelerate market expansion, while investments by leading medical device companies foster continuous innovation. Hospitals and clinics are increasingly prioritizing advanced cardiac care, resulting in higher demand for electrophysiology procedures. Trends such as the integration of digital health tools, the use of artificial intelligence for arrhythmia detection, and growing awareness about early diagnosis contribute to market momentum. Moreover, collaborations between Japanese healthcare providers and global manufacturers help introduce cutting-edge solutions tailored to local clinical needs, strengthening the overall market landscape. As a result, Japan remains a significant hub for electrophysiology device adoption in the Asia-Pacific region.
The Japan Electrophysiology Devices Market demonstrates strong development across major regions, with Kanto and Kansai serving as leading hubs due to their advanced healthcare infrastructure and concentration of specialized cardiac centers. Urban regions such as Chubu and Kyushu also display notable growth, fueled by investments in hospital technology and expanded access to electrophysiology procedures. Regional hospitals and clinics are steadily adopting state-of-the-art diagnostic and ablation devices to address the rising incidence of cardiac arrhythmias among an aging population. Key players driving the market’s progress include Biosense Webster Inc., a pioneer in advanced mapping and ablation technologies; Medtronic PLC, recognized for its wide-ranging electrophysiology product offerings; and Nihon Kohden Corporation, a prominent domestic manufacturer providing innovative cardiac monitoring solutions. These companies, along with others such as Abbott Laboratories, contribute to a highly competitive and innovation-driven market environment in Japan.
Market Insights
- The Japan Electrophysiology Devices Market is projected to grow from USD 794.06 million in 2024 to USD 2,470.07 million by 2032, registering a CAGR of 15.24%.
- Rising prevalence of cardiac arrhythmias and an aging population drive steady market demand for advanced electrophysiology devices.
- Hospitals and clinics are rapidly adopting minimally invasive procedures and digital health tools to improve patient outcomes and streamline care delivery.
- Key players such as Biosense Webster Inc., Medtronic PLC, Nihon Kohden Corporation, and Abbott Laboratories foster a competitive landscape through continuous innovation and product development.
- Complex regulatory and reimbursement environments present significant barriers for new entrants and can delay the introduction of novel technologies to the market.
- The Kanto and Kansai regions lead in device adoption, supported by extensive healthcare infrastructure and high procedural volumes, while Chubu and Kyushu regions experience steady growth due to healthcare modernization and public health initiatives.
- Increasing collaboration between domestic healthcare providers and global device manufacturers accelerates the introduction of advanced solutions, helping the market maintain a dynamic and innovative trajectory.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers
Growing Prevalence of Cardiac Arrhythmias Fuels Demand for Advanced Solutions
The increasing incidence of cardiac arrhythmias, including atrial fibrillation and ventricular tachycardia, remains a primary growth driver for the Japan Electrophysiology Devices Market. Lifestyle changes, rising rates of hypertension, and a rapidly aging population contribute to a higher prevalence of heart rhythm disorders across the country. This surge in patient volume creates robust demand for advanced diagnostic and therapeutic electrophysiology devices. Hospitals and clinics expand their capabilities by investing in state-of-the-art mapping systems and ablation catheters to address this growing patient need. The emphasis on early detection and management of arrhythmias ensures a steady pipeline of procedures. These trends support the market’s strong growth outlook and highlight the importance of technological innovation in cardiac care.
- For instance, cardiovascular diseases account for approximately 15% of all deaths in Japan, with atrial fibrillation affecting nearly 1 million people nationwide.
Government Initiatives and Reimbursement Policies Strengthen Market Growth
Japan’s healthcare system provides substantial support for cardiac care through favorable government initiatives and reimbursement frameworks. Policies targeting the early diagnosis and treatment of heart rhythm disorders enable hospitals to adopt advanced electrophysiology technologies without excessive financial burden. It benefits from clear regulatory pathways and national health insurance coverage for key procedures, making innovative solutions more accessible to both patients and providers. Collaborative programs between public institutions and device manufacturers promote the development and adoption of high-quality medical devices. The combination of supportive regulation and funding creates a conducive environment for rapid market expansion. Stakeholders across the ecosystem benefit from reduced barriers to entry and streamlined device deployment.
- For instance, Japan’s national health insurance system covers a significant portion of electrophysiology procedures, making advanced cardiac care more accessible.
Technological Advancements Improve Clinical Outcomes and Efficiency
Ongoing advancements in electrophysiology technology transform patient care and procedural success rates within the Japan Electrophysiology Devices Market. Enhanced 3D mapping systems, integration of artificial intelligence, and the introduction of next-generation ablation tools allow clinicians to perform complex procedures with higher accuracy and reduced risk. Hospitals achieve greater workflow efficiency and improved patient outcomes by utilizing these innovative devices. Medical professionals gain confidence in adopting new methods due to improved reliability and ease of use. The push for less invasive procedures aligns with patient preferences and accelerates the adoption of cutting-edge devices. Technological evolution remains a critical factor driving the market forward.
Strong Collaboration and Investment from Key Industry Stakeholders
Collaboration between domestic healthcare providers and global medical device manufacturers propels the growth of the Japan Electrophysiology Devices Market. Investments in research, clinical training, and product customization ensure that devices meet the specific requirements of Japanese patients and clinicians. International partnerships facilitate the transfer of knowledge and the introduction of global best practices to the local market. Manufacturers invest in marketing, training, and after-sales support to build long-term relationships with leading hospitals. Active participation from both public and private sectors sustains momentum for continuous market innovation. These partnerships help establish Japan as a leading destination for advanced electrophysiology procedures within the Asia-Pacific region.
Market Trends
Adoption of Minimally Invasive Electrophysiology Procedures Accelerates
The shift toward minimally invasive procedures stands out among the leading trends in the Japan Electrophysiology Devices Market. Hospitals and clinics continue to invest in advanced catheters and mapping systems that support shorter recovery times and improved patient comfort. Minimally invasive techniques gain preference due to their lower complication rates and enhanced procedural precision. Patients and physicians alike recognize the benefits of reduced hospital stays and quicker returns to daily activities. This trend aligns with Japan’s focus on improving healthcare efficiency and patient satisfaction. The market sees increased adoption of technologies that enable safer and more effective interventions for cardiac arrhythmias.
- For instance, minimally invasive cardiac procedures have increased by 20% in Japan over the past five years, driven by advancements in catheter-based treatments.
Integration of Digital Health and Artificial Intelligence Enhances Care Quality
Digital health tools and artificial intelligence become increasingly influential in shaping the Japan Electrophysiology Devices Market. AI-driven mapping and diagnostic systems offer real-time insights that enable clinicians to detect and treat arrhythmias with greater accuracy. The integration of digital platforms into electrophysiology workflows supports data-driven decision-making and facilitates remote monitoring of patients. Hospitals deploy these solutions to enhance clinical outcomes and streamline care delivery. Telemedicine adoption further extends the reach of specialist care, benefiting patients in remote areas. The trend toward digitalization establishes a foundation for personalized and connected cardiac care across the country.
- For instance, Japan’s digital health market is projected to grow significantly, with AI-driven solutions playing a key role in cardiac care.
Focus on Early Detection and Preventive Cardiology Drives Innovation
Rising awareness about the importance of early detection fuels demand for advanced screening and diagnostic tools in the Japan Electrophysiology Devices Market. Healthcare providers promote regular heart rhythm monitoring and risk assessment for at-risk populations, leveraging portable and wearable devices. The emphasis on preventive cardiology drives investments in solutions that enable timely intervention and management of arrhythmias. Device manufacturers respond with innovative products designed for user convenience and reliability. Early diagnosis and proactive treatment help reduce long-term healthcare costs and improve patient outcomes. This trend reflects a shift toward more comprehensive and preventive approaches in cardiovascular care.
Collaborative Ecosystem Supports Continuous Product Development
The collaborative environment among healthcare providers, academic institutions, and industry players supports ongoing product development in the Japan Electrophysiology Devices Market. Manufacturers partner with leading hospitals and universities to conduct clinical research, test new technologies, and gather real-world evidence. These collaborations facilitate the customization of devices to meet the specific needs of Japanese clinicians and patients. The ecosystem encourages the rapid adoption of innovative solutions and accelerates their introduction to the market. Government support for research and development further strengthens this trend. The result is a dynamic and responsive market, positioned at the forefront of global electrophysiology advancements.
Market Challenges Analysis
Regulatory and Reimbursement Complexity Creates Barriers to Market Entry
Navigating Japan’s regulatory environment presents significant challenges for companies operating in the Japan Electrophysiology Devices Market. The approval process for new devices can be lengthy and requires extensive clinical evidence to demonstrate safety and efficacy. Delays in regulatory approvals often hinder the timely introduction of innovative solutions to the market. The complexity of reimbursement policies can further limit patient access to advanced devices, impacting sales growth. Smaller companies face resource constraints in meeting these stringent requirements, which can restrict competition and slow innovation. Market participants must develop comprehensive regulatory strategies to ensure successful product launches.
- For instance, Japan’s medical device approval process can take up to five years, delaying the introduction of innovative electrophysiology solutions.
Workforce Limitations and High Capital Costs Hinder Broader Adoption
A shortage of skilled electrophysiologists and support staff limits the capacity of hospitals to offer specialized procedures across Japan. The high capital investment required for advanced electrophysiology equipment and infrastructure can deter smaller healthcare facilities from adopting new technologies. It challenges broader market penetration, especially outside major urban centers. Continuous staff training is necessary to maximize the benefits of technological advancements, but such programs require time and resources. Cost pressures on hospitals may lead to prioritization of other healthcare needs over electrophysiology device upgrades. Addressing these workforce and financial barriers is essential for expanding access and realizing the full potential of market growth.
Market Opportunities
Expansion of Telemedicine and Remote Monitoring Broadens Access to Care
The rapid advancement of telemedicine and remote patient monitoring creates significant opportunities for the Japan Electrophysiology Devices Market. Hospitals and clinics deploy digital platforms to facilitate remote consultations and continuous monitoring of cardiac patients, expanding access beyond major urban centers. Patients benefit from timely interventions and reduced need for in-person visits, which enhances convenience and overall health outcomes. Device manufacturers can leverage this trend by developing electrophysiology solutions compatible with remote data sharing and integration into telehealth systems. The shift toward digital healthcare aligns with national strategies to address demographic changes and healthcare resource limitations. Widespread adoption of telemedicine-supported devices positions the market for sustained long-term growth.
Growing Focus on Preventive Cardiology and Early Diagnosis Fuels Innovation
Increased awareness of preventive cardiology and the importance of early arrhythmia detection presents growth avenues for the Japan Electrophysiology Devices Market. Healthcare providers emphasize routine cardiac screening and risk assessment, supporting demand for user-friendly wearable and portable monitoring devices. Early identification of heart rhythm disorders enables prompt intervention, reducing the burden of advanced cardiac disease. Medical device companies can seize this opportunity by offering innovative, reliable products designed for everyday use and home monitoring. Collaboration with healthcare organizations to promote public education and outreach will further strengthen market adoption. This proactive approach to cardiac care drives both market expansion and improved patient outcomes.
Market Segmentation Analysis:
By Type:
The Japan Electrophysiology Devices Market includes a broad range of device types, each supporting critical aspects of cardiac arrhythmia diagnosis and treatment. Ablation catheters represent a significant market segment, with sub-categories such as radiofrequency (RF) ablation, cryoablation, and pulse field ablation. RF ablation leads in usage, favored for its established efficacy and procedural safety, while cryoablation and pulse field ablation gain traction for specific patient populations and emerging clinical benefits. Diagnostic catheters play a pivotal role in precise mapping and identification of abnormal electrical pathways, supporting accurate treatment planning. Laboratory devices, including advanced mapping systems and recording equipment, form the technological backbone of electrophysiology procedures, helping clinicians improve workflow efficiency and diagnostic accuracy. Access devices enable safe and effective vascular entry, essential for both diagnostic and interventional procedures, thus holding a steady share in the market.
By Indication:
The market divides into atrial fibrillation (AF) and non-atrial fibrillation categories. Atrial fibrillation accounts for a substantial share, reflecting its high prevalence and the growing focus on early and effective intervention. Non-atrial fibrillation indications, such as supraventricular tachycardia and ventricular tachycardia, also contribute steadily to demand, supported by the expansion of advanced treatment protocols in Japanese healthcare. Device manufacturers address both categories with innovations tailored to distinct arrhythmia types, broadening the market’s clinical relevance.
By End-Use:
Inpatient facilities dominate the Japan Electrophysiology Devices Market, driven by their access to advanced equipment, skilled specialists, and comprehensive cardiac care infrastructure. Outpatient facilities experience steady growth, supported by the trend toward minimally invasive procedures and the healthcare system’s efforts to manage costs and enhance patient convenience. The “others” segment includes research centers, academic hospitals, and specialized clinics, which contribute to technology adoption and clinical research. Each segment, defined by type, indication, and end-use, shapes a dynamic market landscape that continues to evolve in response to shifting clinical, technological, and demographic factors in Japan.
Segments:
Based on Type:
- Ablation Catheters
- Radiofrequency (RF) Ablation
- Cryoablation
- Pulse Field Ablation
- Diagnostic Catheters
- Laboratory Devices
- Access Devices
Based on Indication:
- Atrial Fibrillation (AF)
- Non-Atrial Fibrillation
Based on End-Use:
- Inpatient Facilities
- Outpatient Facilities
- Others
Based on the Geography:
- Kanto Region
- Kansai Region
- Chubu Region
- Kyushu Region
- Other Regions
Regional Analysis
Kanto Region
The Kanto region leads the Japan Electrophysiology Devices Market, accounting for approximately 40% of the total market share. This dominance is attributed to the region’s concentration of advanced medical institutions, research centers, and teaching hospitals, particularly in metropolitan Tokyo and its surrounding prefectures. The presence of renowned hospitals and a large patient pool drives high procedural volumes, creating a robust demand for both diagnostic and ablation devices. Leading medical universities and academic hospitals foster innovation and facilitate the early adoption of next-generation electrophysiology technologies. Healthcare providers in Kanto maintain access to significant funding and actively participate in clinical trials and research collaborations, accelerating the introduction of advanced devices. The region’s aging population and high awareness of cardiac health further fuel demand for arrhythmia diagnosis and treatment solutions. The Kanto market benefits from efficient infrastructure and strong relationships with global device manufacturers, ensuring that new product launches and technological upgrades quickly reach clinicians and patients.
Kansai Region
The Kansai region commands a market share of around 23%, making it the second-largest contributor to the Japan Electrophysiology Devices Market. Major cities such as Osaka, Kyoto, and Kobe are home to several leading hospitals and specialty heart centers that support high procedural volumes. Healthcare facilities in Kansai continue to invest in minimally invasive electrophysiology technologies and state-of-the-art laboratory equipment. The region’s established network of university hospitals and research organizations creates an environment that encourages clinical trials, technology evaluation, and professional training. Kansai’s diverse demographic profile, including a significant elderly population, drives sustained demand for arrhythmia management solutions. The availability of experienced electrophysiologists and well-developed healthcare infrastructure further reinforces the region’s strong market position. Kansai often serves as a proving ground for new device launches, with leading hospitals participating in pilot programs and early-phase studies.
Chubu Region
The Chubu region holds about 15% market share in the Japan Electrophysiology Devices Market. Anchored by urban centers such as Nagoya and Shizuoka, Chubu’s market expansion is driven by increased investment in cardiac care infrastructure and growing adoption of advanced electrophysiology procedures. Hospitals and specialty clinics in the region are focusing on integrating digital health solutions and modern laboratory systems to meet rising demand. The region benefits from government initiatives aimed at enhancing regional healthcare quality, which include funding support for medical technology upgrades. Although Chubu has a smaller population base than Kanto or Kansai, its ongoing healthcare modernization efforts and partnerships with medical device companies are narrowing the gap. The adoption of wearable cardiac monitors and home-based diagnostic tools further supports the region’s steady market growth.
Kyushu Region
Kyushu accounts for an estimated 12% of the Japan Electrophysiology Devices Market, supported by a network of major hospitals in cities like Fukuoka and Kumamoto. Healthcare providers in Kyushu are adopting a range of electrophysiology solutions to address the needs of a growing elderly population. The regional government invests in public health campaigns and chronic disease management programs, which increase awareness of cardiac conditions and stimulate demand for diagnostic and interventional devices. Kyushu’s healthcare facilities are also increasingly involved in national and international research collaborations, which drive access to advanced devices and expertise. The expansion of remote and telehealth services is improving access to specialist cardiac care, especially in rural and remote parts of the region. Device manufacturers view Kyushu as a strategic market for launching pilot initiatives and building relationships with local clinicians.
Other Regions
Other regions in Japan collectively account for approximately 10% of the total market share in the Japan Electrophysiology Devices Market. These areas, which include Hokkaido, Tohoku, Shikoku, and various rural prefectures, are characterized by smaller healthcare networks and lower procedural volumes compared to the major metropolitan areas. Nonetheless, government efforts to bridge regional healthcare disparities have resulted in increased investment in hospital infrastructure and medical technology. Smaller hospitals and clinics are gradually upgrading their electrophysiology capabilities, often leveraging telemedicine to connect with specialists in larger cities. Public health initiatives encourage early diagnosis and management of cardiac arrhythmias in these communities, helping to drive market adoption. While growth rates may lag behind the leading regions, sustained support for technology diffusion ensures that these areas remain a vital part of the national market landscape.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Mindray
- Biosense Webster Inc.
- Medtronic PLC
- Abbott Laboratories
- Boston Scientific Corp
- Biotronik AG
- Siemens Healthcare AG
- MicroPort Scientific Corporation
- Koninklijke Philips N.V.
- Nihon Kohden Corporation
- Omron Corporation
- Shimadzu Corporation
- Mitsubishi Electric Corporation
Competitive Analysis
The Japan Electrophysiology Devices Market features an intensely competitive environment shaped by both global leaders and strong domestic players. Leading companies such as Biosense Webster Inc., Medtronic PLC, Abbott Laboratories, Boston Scientific Corp, Nihon Kohden Corporation, Mindray, Biotronik AG, Siemens Healthcare AG, MicroPort Scientific Corporation, Koninklijke Philips N.V., Omron Corporation, Shimadzu Corporation, and Mitsubishi Electric Corporation hold significant positions through their diverse product portfolios and consistent innovation. These players invest heavily in research and development to launch next-generation mapping systems, advanced ablation catheters, and digital health-integrated solutions that enhance procedural efficiency and clinical outcomes. Product differentiation is achieved by integrating digital health solutions, artificial intelligence, and user-friendly interfaces to enhance clinical outcomes and procedural efficiency. Collaboration with hospitals, academic institutions, and research centers enables continuous product improvement, local adaptation, and the development of tailored solutions that meet specific clinical needs. Market participants prioritize robust after-sales service and technical support to strengthen customer relationships and ensure long-term loyalty. Regulatory expertise and a thorough understanding of local reimbursement policies are crucial for sustaining growth and securing product approvals. The competitive landscape encourages ongoing innovation, ensuring that electrophysiology devices in Japan consistently reflect the latest standards in safety, accuracy, and effectiveness.
Recent Developments
- In August 2023, Biosense Webster received approval for various atrial fibrillation ablation products that can be utilized in a workflow without fluoroscopy during catheter ablation procedures.
- In August 2023, Boston Scientific Corporation (US) launched the POLARx cryoablation system. This system is used to treat patients with paroxysmal atrial fibrillation.
- In May 2023 Abbott Laboratories launched the Tactiflex ablation catheter which is sensor-enabled and it is used to treat the most common abnormal heart rhythm.
Market Concentration & Characteristics
The Japan Electrophysiology Devices Market exhibits a moderate to high level of market concentration, with a mix of established international corporations and prominent domestic manufacturers dominating the landscape. It features strong barriers to entry due to stringent regulatory requirements, high capital investment, and the need for specialized clinical expertise. Leading companies maintain their positions by offering comprehensive product portfolios, continuous innovation, and robust after-sales support. The market demonstrates a strong focus on technological advancement, digital integration, and clinical precision, driven by Japan’s aging population and demand for minimally invasive cardiac procedures. Hospitals and healthcare providers in major metropolitan areas demonstrate higher adoption rates for advanced devices, while regional facilities gradually expand their capabilities. The dynamic environment ensures ongoing improvements in device safety, accuracy, and patient outcomes, establishing a foundation for sustained market growth.
Report Coverage
The research report offers an in-depth analysis based on Type, Indication, End-Use and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market will continue to grow rapidly, driven by increased awareness and diagnosis of cardiac arrhythmias.
- Hospitals and clinics are expected to adopt more advanced and minimally invasive electrophysiology devices.
- Digital health integration and artificial intelligence will enhance procedure accuracy and patient monitoring.
- Research and development efforts will introduce new mapping systems and ablation technologies.
- Strategic collaborations between local healthcare providers and global manufacturers will accelerate technology transfer.
- Regulatory pathways may streamline to support faster adoption of innovative devices.
- Training programs and professional development will expand to address the shortage of skilled electrophysiologists.
- Remote monitoring and telemedicine solutions will see broader adoption for arrhythmia management.
- Market competition will intensify, encouraging price competitiveness and continuous product improvement.
- Patient-centric care models and early detection initiatives will remain central to long-term market growth.




