Market Overview
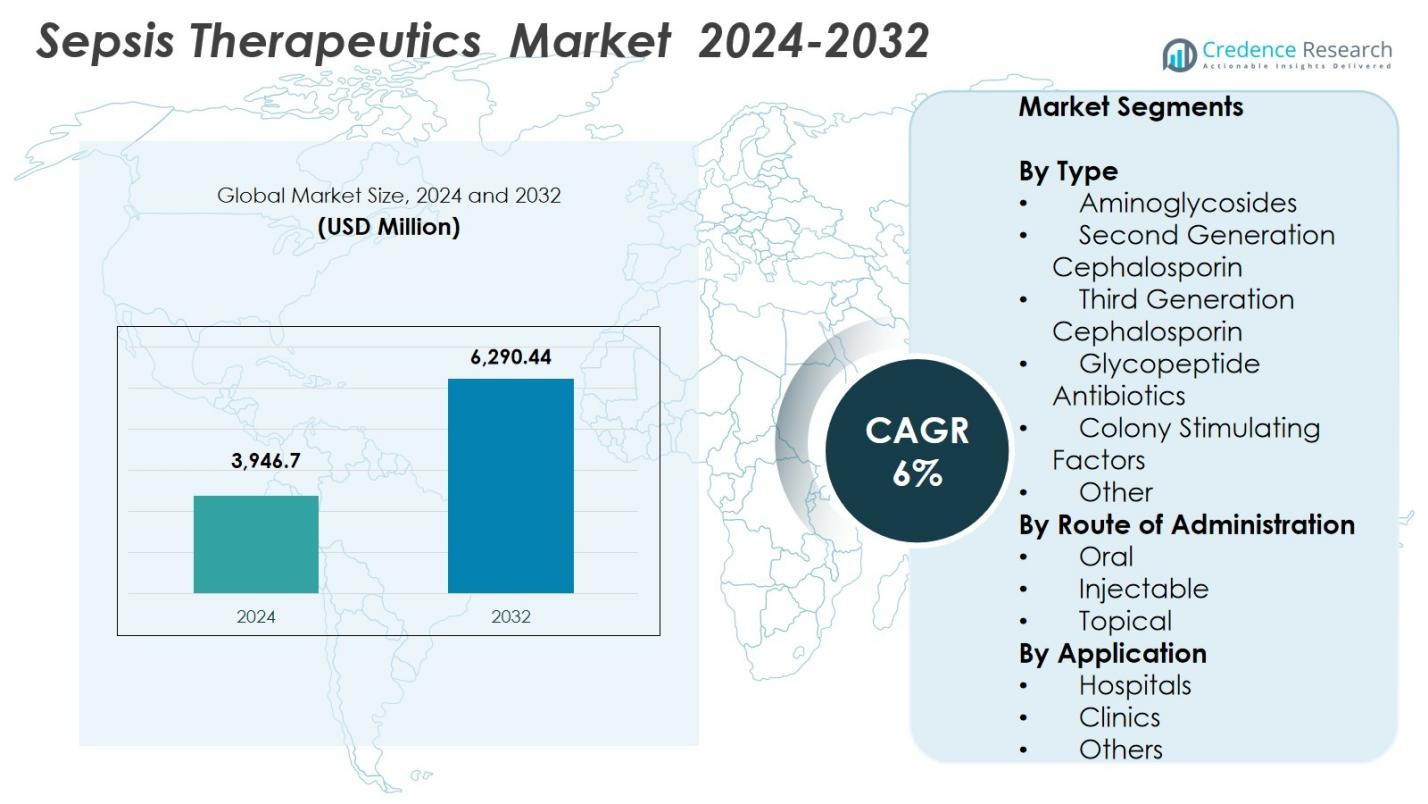
Sepsis Therapeutics Market size was valued at USD 3,946.7 Million in 2024 and is anticipated to reach USD 6,290.44 Million by 2032, at a CAGR of 6% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Sepsis Therapeutics Market Size 2024 |
USD 3,946.7 Million |
| Sepsis Therapeutics Market, CAGR |
6% |
| Sepsis Therapeutics Market Size 2032 |
USD 6,290.44 Million |
The Sepsis Therapeutics Market is driven by strong participation from leading players such as Eli Lilly and Company, AstraZeneca, Bayer AG, Gilead Sciences, Abbott Laboratories, La Jolla Pharmaceutical Company, Agennix, Atox Bio, CytoGenix, and TaiRx, Inc., all of which are advancing antimicrobial, immunomodulatory, and biologic treatment portfolios. These companies focus on addressing rising antimicrobial resistance and improving clinical outcomes through innovative drug pipelines and strategic partnerships. Regionally, North America led the market with a 38.4% share in 2024, supported by advanced critical care infrastructure, high treatment adoption, and strong R&D activity, positioning it as the dominant hub for sepsis therapeutic development.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The Sepsis Therapeutics Market was valued at USD 3,946.7 million in 2024 and is projected to reach USD 6,290.44 million by 2032, growing at a CAGR of 6%.
- Market growth is driven by rising sepsis incidence, expanding ICU admissions, and increased adoption of advanced antimicrobial and immunomodulatory therapies across hospitals.
- Key trends include rapid adoption of precision medicine, AI-assisted diagnostic tools, and accelerated development of next-generation antibiotics targeting resistant pathogens.
- Major players such as Eli Lilly and Company, AstraZeneca, Bayer AG, Gilead Sciences, Abbott Laboratories, and La Jolla Pharmaceutical Company are strengthening pipelines through R&D investments and strategic partnerships.
- North America dominated with 38.4% share in 2024, while hospitals led the application segment with 69.8% share; Asia-Pacific is the fastest-growing region due to rising infection rates and expanding healthcare infrastructure.
Market Segmentation Analysis
By Type
The Sepsis Therapeutics market by type was led by third-generation cephalosporins, accounting for an 34.6% share in 2024, driven by their broad-spectrum activity and effectiveness against multidrug-resistant gram-negative pathogens. Their rapid bactericidal action and proven clinical efficacy in severe sepsis cases support widespread hospital adoption. Aminoglycosides and glycopeptide antibiotics also show strong demand due to rising antimicrobial resistance, while colony-stimulating factors gain traction for improving immune recovery in septic patients. Increasing clinical trials and enhanced treatment protocols continue to boost adoption across all therapeutic classes.
- For instance, Ceftazidime‑Avibactam a third-generation cephalosporin combined with a β-lactamase inhibitor showed 75.4% clinical efficacy and a 66.0% bacterial clearance rate in a multicenter retrospective study of 183 patients with carbapenem-resistant gram-negative infections, with only 11.5% 30-day all-cause mortality.
By Route of Administration
The injectable segment dominated the Sepsis Therapeutics market with 72.3% share in 2024, reflecting the urgent nature of sepsis treatment, which requires rapid drug absorption and intravenous delivery for effective patient stabilization. Injectable formulations remain the standard of care in emergency departments and intensive care units, supported by their fast onset of action and controlled dosing. Oral therapies account for a smaller share due to limited suitability for critical cases, while topical routes remain niche for localized infections. Increasing hospitalizations and advanced IV antibiotic therapies continue to strengthen the injectable segment.
- For instance, Paratek’s NUZYRA (omadacycline) IV formulation continued adoption in hospitals following clinical results showing comparable efficacy to moxifloxacin in treating community-acquired bacterial infections, supporting its role when rapid parenteral initiation is required before transitioning to oral dosing.
By Application
Hospitals remained the leading application segment in 2024, capturing 69.8% market share, driven by the high volume of sepsis cases requiring intensive monitoring, advanced diagnostics, and immediate therapeutic intervention. The strong presence of critical care units, availability of trained professionals, and integration of standardized sepsis management protocols further enhance hospital demand. Clinics account for moderate usage, primarily for follow-up treatment and early-stage infection management, while the “others” category includes ambulatory centers and long-term care facilities. Rising global sepsis burden and improved hospital infrastructure continue to support segment dominance.
Key Growth Drivers
Advancements in Antimicrobial and Adjunctive Therapies
Rapid advancements in antimicrobial formulations and adjunctive therapies are significantly accelerating growth in the Sepsis Therapeutics market. New generations of broad-spectrum antibiotics, anti-endotoxin agents, and immunomodulators are improving survival outcomes and reducing organ dysfunction in severe sepsis. Pharmaceutical companies are increasingly investing in molecules targeting resistant gram-negative bacteria, addressing one of the most persistent clinical challenges. Additionally, biologics such as cytokine inhibitors and colony-stimulating factors are expanding therapeutic options beyond traditional antibiotics. The rise in adaptive clinical trial designs and expedited regulatory pathways for life-threatening infections further supports faster commercialization of innovative therapies.
- For instance, Xacduro (sulbactam + durlobactam) approved by U.S. Food and Drug Administration (FDA) in May 2023 for serious hospital-acquired and ventilator-associated pneumonia caused by carbapenem-resistant Acinetobacter baumannii
Rising Prevalence of Sepsis and Growing Hospital Admissions
The global rise in sepsis cases, driven by aging populations, chronic disease prevalence, and increased hospital-acquired infections, remains a major market growth catalyst. High-risk groups—including elderly patients, immunocompromised individuals, and those undergoing invasive surgeries—are contributing to escalating sepsis incidence. Hospitals worldwide are reporting higher ICU admissions linked to severe bacterial, viral, and fungal infections, boosting demand for advanced sepsis treatments. Increased awareness campaigns and improved clinical recognition of early sepsis symptoms are enhancing diagnosis rates. Moreover, post-pandemic healthcare reforms have strengthened critical care capacity, creating sustained demand for effective sepsis therapeutics across emergency settings.
- For instance, a U.S. CDC analysis noted that nearly 87% of sepsis cases originate in the community but require hospitalization, and about 1 in 3 patients who die in a hospital have sepsis, underscoring rising ICU and emergency department pressures.
Expansion of Diagnostic Capabilities and Early Intervention Protocols
Enhanced diagnostic capabilities and early intervention protocols are fundamentally reshaping treatment efficiency and driving therapeutic uptake. Rapid molecular diagnostics, point-of-care tests, and biomarker-based assays enable earlier detection of bloodstream infections, allowing physicians to initiate targeted therapy within critical time windows. Hospitals are increasingly adopting standardized sepsis management bundles, integrating tools such as lactate monitoring and automated alert systems. These advancements reduce mortality rates and minimize antibiotic misuse, reinforcing the need for timely therapeutic administration. As healthcare systems prioritize early intervention to improve outcomes and reduce treatment costs, demand for reliable and fast-acting sepsis drugs continues to expand.
Key Trends & Opportunities
Development of Precision and Personalized Sepsis Therapies
Precision medicine is emerging as a transformative trend in the Sepsis Therapeutics market, offering opportunities to tailor treatments based on patient-specific immune responses, genetic markers, and pathogen profiles. Researchers are developing host-directed therapeutics that modulate immune dysregulation, a central factor in sepsis progression. Pharmacogenomic screening is improving drug selection and reducing adverse reactions, while machine learning models predict patient trajectories to guide individualized therapy. This shift toward personalized treatment strategies opens new avenues for biologics, immunotherapies, and companion diagnostics. Companies leveraging these innovations are positioned to address unmet needs in severe and refractory sepsis cases.
- For instance, Inflammatix created the TriVerity test, an FDA-cleared host-response diagnostic using AI to interpret 29 mRNA markers from blood samples, providing scores for bacterial/viral likelihood and infection severity to inform personalized therapy decisions in acute care.
Integration of AI, Predictive Analytics, and Digital Health Tools
The integration of AI-driven predictive analytics and digital health platforms presents significant opportunities for enhancing sepsis treatment effectiveness. AI-enabled clinical decision support tools can identify sepsis risk early, optimize antibiotic selection, and forecast treatment responses using real-time patient data. Remote monitoring technologies and connected ICU systems allow clinicians to track vital signs continuously, enabling proactive interventions. Digital platforms also support better stewardship of antibiotics by evaluating resistance patterns and adjusting dosing protocols. As healthcare providers shift toward data-driven care, demand for therapeutics that align with advanced monitoring and predictive tools is expected to grow rapidly.
- For instance, connected ICU platforms such as Philips’ IntelliVue Guardian demonstrated reduced code-blue events in clinical evaluations by using continuous remote monitoring and early-warning algorithms that alert clinicians before patient deterioration.
Key Challenges
Escalating Antimicrobial Resistance Impacting Treatment Outcomes
Antimicrobial resistance (AMR) remains one of the most significant challenges restraining the Sepsis Therapeutics market. Rising resistance among gram-negative bacteria, including carbapenem-resistant Enterobacteriaceae and multidrug-resistant Pseudomonas species, severely limits the effectiveness of existing antibiotics. This forces clinicians to rely on older, more toxic drugs or untested combinations, raising safety concerns and treatment complexities. AMR-driven clinical failures are increasing ICU stays, mortality rates, and overall healthcare costs. While new antibiotic development is underway, the discovery pipeline remains insufficient compared to the pace of resistance. Addressing AMR requires coordinated global strategies, antibiotic stewardship programs, and sustained R&D investment.
High Treatment Costs and Limited Access in Low-Resource Settings
High treatment costs pose a major barrier to widespread adoption of advanced sepsis therapeutics, particularly in low- and middle-income countries. Innovative biologics, next-generation antibiotics, and advanced diagnostics significantly increase overall treatment expenditure, limiting affordability for both healthcare systems and patients. In many regions, constraints related to ICU capacity, diagnostic infrastructure, and skilled medical personnel further restrict timely access to sepsis care. Delayed treatment initiation contributes to higher mortality rates and poorer outcomes. These disparities highlight the need for cost-effective therapeutics, expanded healthcare funding, and scalable clinical protocols that can be implemented even in resource-constrained environments.
Regional Analysis
North America
North America dominated the Sepsis Therapeutics market in 2024 with an 38.4% share, driven by high sepsis incidence, advanced critical care infrastructure, and strong adoption of innovative antimicrobial and immunomodulatory therapies. The region benefits from extensive R&D investment, robust antibiotic stewardship programs, and faster regulatory approvals that accelerate drug availability. Growing awareness among clinicians, improved diagnostic capabilities, and rising hospital admissions due to chronic illnesses further strengthen demand. The U.S. leads the regional landscape, supported by major pharmaceutical players, strong reimbursement frameworks, and expanding clinical trials focused on next-generation sepsis treatments.
Europe
Europe held 27.1% share of the Sepsis Therapeutics market in 2024, supported by its well-established healthcare systems, increasing ICU capacity, and strong emphasis on adherence to clinical sepsis management guidelines. The region experiences high sepsis burden due to aging populations and rising antimicrobial resistance, driving demand for advanced therapeutics and rapid diagnostics. Government-funded awareness programs and stringent infection-control protocols further enhance treatment utilization. Countries such as Germany, the U.K., and France lead adoption, fueled by continuous research collaborations and favorable regulatory initiatives that support the development of novel sepsis treatment solutions.
Asia-Pacific
Asia-Pacific accounted for 22.8% share in 2024 and is expected to grow at the fastest pace due to rising infection rates, expanding hospital infrastructure, and increasing healthcare expenditure across emerging economies. High sepsis prevalence among neonatal and elderly populations drives significant therapeutic demand. Governments in China, India, and Southeast Asia are investing in critical care upgrades, antibiotic accessibility, and sepsis awareness programs. The region also benefits from improving diagnostic adoption and growing pharmaceutical manufacturing capabilities. Rapid urbanization, rising chronic disease incidence, and enhanced emergency care availability support strong future growth prospects.
Latin America
Latin America captured an 6.3% market share in 2024, influenced by rising sepsis-related hospitalizations, improving access to essential antibiotics, and ongoing expansion of public healthcare systems. Countries such as Brazil and Mexico are investing in enhanced ICU infrastructure and sepsis management guidelines to reduce mortality rates. However, uneven healthcare quality and limited availability of advanced biologics constrain broader adoption. International partnerships and government-led infection-control initiatives are gradually improving diagnostic and treatment capacities, positioning the region for moderate growth as healthcare modernization efforts gain momentum.
Middle East & Africa
The Middle East & Africa region held 5.4% share of the Sepsis Therapeutics market in 2024, driven by improving healthcare infrastructure, rising awareness of sepsis mortality, and increasing investments in critical care facilities. Gulf countries, including Saudi Arabia and the UAE, are leading adoption due to strong healthcare spending and availability of advanced therapeutics. In contrast, many African nations face challenges such as limited ICU capacity, diagnostic shortages, and affordability issues, resulting in delayed treatment initiation. International aid programs, expanded training initiatives, and gradual adoption of essential sepsis protocols are helping strengthen regional treatment capabilities.
Market Segmentations
By Type
- Aminoglycosides
- Second Generation Cephalosporin
- Third Generation Cephalosporin
- Glycopeptide Antibiotics
- Colony Stimulating Factors
- Other
By Route of Administration
By Application
By Geography
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Competitive Landscape
The Sepsis Therapeutics market features a diverse group of global and emerging companies focusing on antimicrobial innovation, immunomodulatory drug development, and advanced biologics. Key players such as Eli Lilly and Company, AstraZeneca, Bayer AG, Gilead Sciences, Abbott Laboratories, La Jolla Pharmaceutical Company, Agennix, Atox Bio, CytoGenix, and TaiRx, Inc. are actively expanding their clinical pipelines to address rising antimicrobial resistance and unmet medical needs in severe sepsis. Many companies are investing in next-generation antibiotics, host-directed therapies, and anti-inflammatory agents to improve survival outcomes. Strategic collaborations, accelerated regulatory pathways, and increasing Phase II/III clinical trials are shaping the market’s growth dynamics. Emerging biotech firms are contributing to niche innovations, particularly in immunotherapy and targeted biologics. As healthcare systems prioritize early intervention and improved treatment efficacy, companies with robust R&D capabilities, strong commercialization networks, and diversified sepsis portfolios are strengthening their market presence.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
Recent Developments
- In December 2025, VolitionRx Limited announced that its Nu.Q® NETs H3.1 assay was included in a French “real-world evaluation” program for early detection of sepsis, as part of a national initiative under the DETECSEPS Consortium.
- In November 2025, ABIONYX Pharma entered advanced strategic discussions with IHU SEPSIS, aiming to build a global integrated platform for sepsis treatment combining apoA-I based therapeutics and HDL-derived vectors.
- In November 2025, ABIONYX Pharma also formed an exclusive global strategic partnership with SEBIA to validate new infectious and metabolic diagnostic tests allowing earlier and more accurate identification of sepsis severity.
Report Coverage
The research report offers an in-depth analysis based on Type, Route of Administration, Application, and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market will experience steady growth driven by rising global sepsis incidence and expanding critical care capacity.
- Development of targeted immunotherapies will strengthen treatment options for severe and refractory sepsis cases.
- Precision medicine approaches will enable more personalized and effective therapeutic interventions.
- AI-powered diagnostic tools will support earlier detection and faster therapeutic decision-making.
- Pharmaceutical pipelines will expand with next-generation antibiotics addressing multidrug-resistant pathogens.
- Collaborations between biotech firms and research institutions will accelerate innovation in adjunctive therapies.
- Greater adoption of rapid molecular diagnostics will improve treatment timing and outcomes.
- Healthcare systems will increase investment in sepsis management protocols and ICU infrastructure.
- Regulatory agencies will continue to provide accelerated pathways for life-saving sepsis therapies.
- Emerging markets will witness increased therapeutic uptake due to improved awareness and healthcare modernization.




