CHAPTER NO. 1 : GENESIS OF THE MARKET
1.1 Market Prelude – Introduction & Scope
1.2 The Big Picture – Objectives & Vision
1.3 Strategic Edge – Unique Value Proposition
1.4 Stakeholder Compass – Key Beneficiaries
CHAPTER NO. 2 : EXECUTIVE LENS
2.1 Pulse of the Industry – Market Snapshot
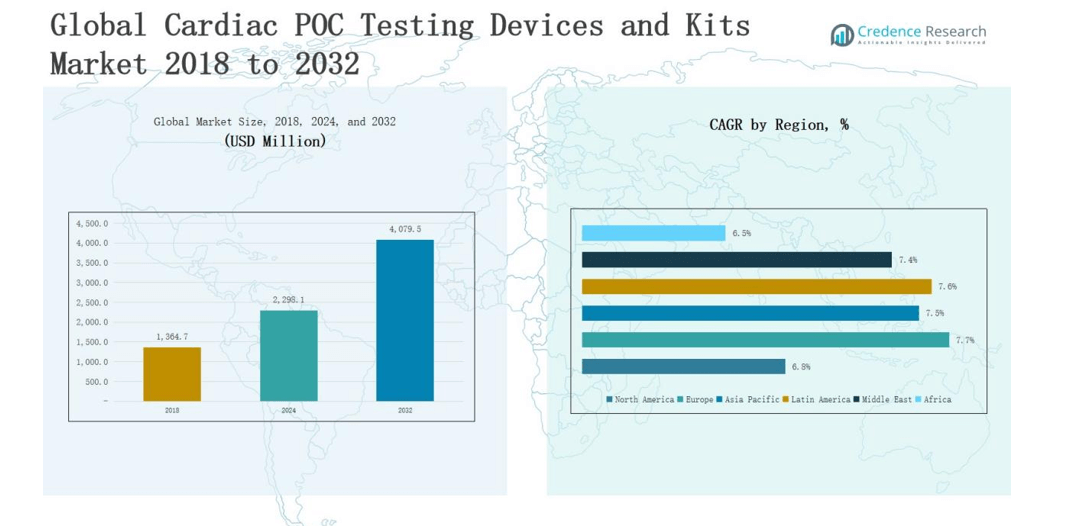
2.2 Growth Arc – Revenue Projections (USD Million)
2.3. Premium Insights – Based on Primary Interviews
CHAPTER NO. 3 : CARDIAC POC TESTING DEVICES AND KITS MARKET FORCES & INDUSTRY PULSE
3.1 Foundations of Change – Market Overview
3.2 Catalysts of Expansion – Key Market Drivers
3.2.1 Momentum Boosters – Growth Triggers
3.2.2 Innovation Fuel – Disruptive Technologies
3.3 Headwinds & Crosswinds – Market Restraints
3.3.1 Regulatory Tides – Compliance Challenges
3.3.2 Economic Frictions – Inflationary Pressures
3.4 Untapped Horizons – Growth Potential & Opportunities
3.5 Strategic Navigation – Industry Frameworks
3.5.1 Market Equilibrium – Porter’s Five Forces
3.5.2 Ecosystem Dynamics – Value Chain Analysis
3.5.3 Macro Forces – PESTEL Breakdown
CHAPTER NO. 4 : KEY INVESTMENT EPICENTER
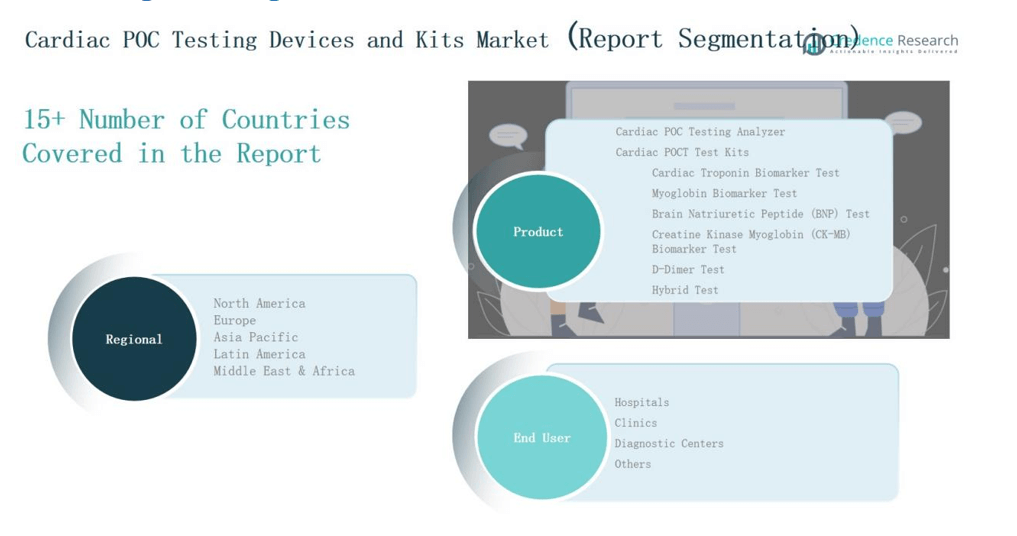
4.1 Regional Goldmines – High-Growth Geographies
4.2 Product Frontiers – Lucrative Product Categories
4.3 End User Sweet Spots – Emerging Demand Segments
CHAPTER NO. 5: REVENUE TRAJECTORY & WEALTH MAPPING
5.1 Momentum Metrics – Forecast & Growth Curves
5.2 Regional Revenue Footprint – Market Share Insights
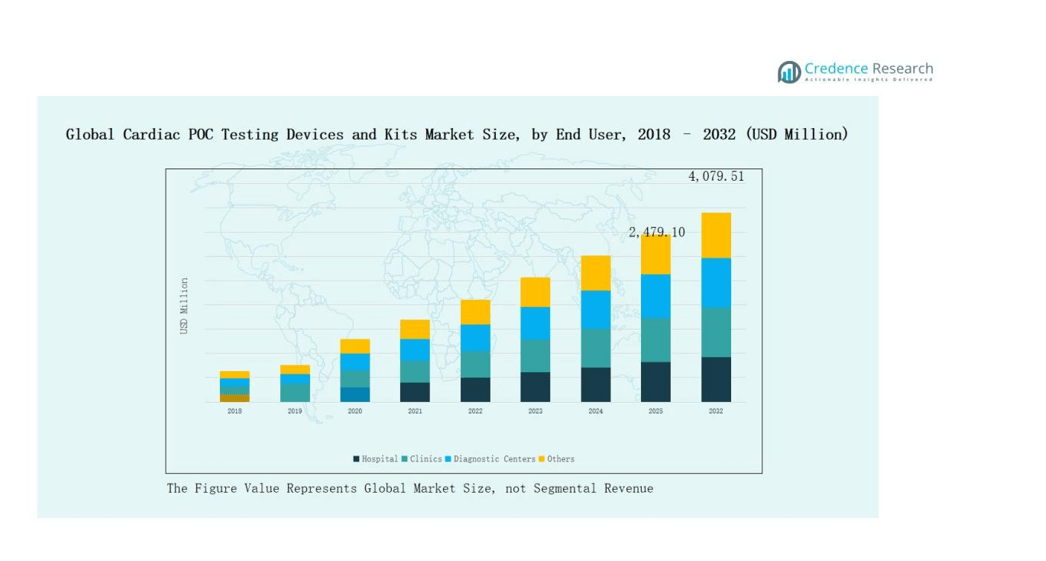
5.3 Segmental Wealth Flow – Product & End User Revenue
CHAPTER NO. 6 : TRADE & COMMERCE ANALYSIS
6.1. Import Analysis By Region
6.1.1. Global Cardiac POC Testing Devices and Kits Market Import Revenue By Region
6.2. Export Analysis By Region
6.2.1. Global Cardiac POC Testing Devices and Kits Market Export Revenue By Region
CHAPTER NO. 7 : COMPETITION ANALYSIS
7.1. Company Market Share Analysis
7.1.1. Global Cardiac POC Testing Devices and Kits Market: Company Market Share
7.2. Global Cardiac POC Testing Devices and Kits Market Company Revenue Market Share
7.3. Strategic Developments
7.3.1. Acquisitions & Mergers
7.3.2. New Product Launch
7.3.3. Regional Expansion
7.4. Competitive Dashboard
7.5. Company Assessment Metrics, 2024
CHAPTER NO. 8 : CARDIAC POC TESTING DEVICES AND KITS MARKET – BY PRODUCT SEGMENT ANALYSIS
8.1. Cardiac POC Testing Devices and Kits Market Overview By Product Segment
8.1.1. Cardiac POC Testing Devices and Kits Market Revenue Share By Product
8.2. Cardiac POC Testing Analyzer
8.3. Cardiac POCT Test Kits
8.3.1. Cardiac Troponin Biomarker Test
8.3.2. Myoglobin Biomarker Test
8.3.3. Brain Natriuretic Peptide (BNP) Test
8.3.4. Creatine Kinase Myoglobin (CK-MB) Biomarker Test
8.3.5. D-Dimer Test
8.3.6. Hybrid Test
CHAPTER NO. 9 : CARDIAC POC TESTING DEVICES AND KITS MARKET – BY END USER SEGMENT ANALYSIS
9.1. Cardiac POC Testing Devices and Kits Market Overview By End User Segment
9.1.1. Cardiac POC Testing Devices and Kits Market Revenue Share By End User
9.2. Hospitals
9.3. Clinics
9.4. Diagnostic Centers
9.5. Others
CHAPTER NO. 10 : CARDIAC POC TESTING DEVICES AND KITS MARKET – REGIONAL ANALYSIS
10.1. Cardiac POC Testing Devices and Kits Market Overview By Region Segment
10.1.1. Global Cardiac POC Testing Devices and Kits Market Revenue Share By Region
10.1.2. Regions
10.1.3. Global Cardiac POC Testing Devices and Kits Market Revenue By Region
10.1.4. Product
10.1.5. Global Cardiac POC Testing Devices and Kits Market Revenue By Product
10.1.6. End User
10.1.7. Global Cardiac POC Testing Devices and Kits Market Revenue By End User
CHAPTER NO. 11 : NORTH AMERICA CARDIAC POC TESTING DEVICES AND KITS MARKET – COUNTRY ANALYSIS
11.1. North America Cardiac POC Testing Devices and Kits Market Overview By Country Segment
11.1.1. North America Cardiac POC Testing Devices and Kits Market Revenue Share By Region
11.2. North America
11.2.1. North America Cardiac POC Testing Devices and Kits Market Revenue By Country
11.2.2. Product
11.2.3. North America Cardiac POC Testing Devices and Kits Market Revenue By Product
11.2.4. End User
11.2.5. North America Cardiac POC Testing Devices and Kits Market Revenue By End User
11.3. U.S.
11.4. Canada
11.5. Mexico
CHAPTER NO. 12 : EUROPE CARDIAC POC TESTING DEVICES AND KITS MARKET – COUNTRY ANALYSIS
12.1. Europe Cardiac POC Testing Devices and Kits Market Overview By Country Segment
12.1.1. Europe Cardiac POC Testing Devices and Kits Market Revenue Share By Region
12.2. Europe
12.2.1. Europe Cardiac POC Testing Devices and Kits Market Revenue By Country
12.2.2. Product
12.2.3. Europe Cardiac POC Testing Devices and Kits Market Revenue By Product
12.2.4. End User
12.2.5. Europe Cardiac POC Testing Devices and Kits Market Revenue By End User
12.3. UK
12.4. France
12.5. Germany
12.6. Italy
12.7. Spain
12.8. Russia
12.9. Rest of Europe
CHAPTER NO. 13 : ASIA PACIFIC CARDIAC POC TESTING DEVICES AND KITS MARKET – COUNTRY ANALYSIS
13.1. Asia Pacific Cardiac POC Testing Devices and Kits Market Overview By Country Segment
13.1.1. Asia Pacific Cardiac POC Testing Devices and Kits Market Revenue Share By Region
13.2. Asia Pacific
13.2.1. Asia Pacific Cardiac POC Testing Devices and Kits Market Revenue By Country
13.2.2. Product
13.2.3. Asia Pacific Cardiac POC Testing Devices and Kits Market Revenue By Product
13.2.4. End User
13.2.5. Asia Pacific Cardiac POC Testing Devices and Kits Market Revenue By End User
13.3. China
13.4. Japan
13.5. South Korea
13.6. India
13.7. Australia
13.8. Southeast Asia
13.9. Rest of Asia Pacific
CHAPTER NO. 14 : LATIN AMERICA CARDIAC POC TESTING DEVICES AND KITS MARKET – COUNTRY ANALYSIS
14.1. Latin America Cardiac POC Testing Devices and Kits Market Overview By Country Segment
14.1.1. Latin America Cardiac POC Testing Devices and Kits Market Revenue Share By Region
14.2. Latin America
14.2.1. Latin America Cardiac POC Testing Devices and Kits Market Revenue By Country
14.2.2. Product
14.2.3. Latin America Cardiac POC Testing Devices and Kits Market Revenue By Product
14.2.4. End User
14.2.5. Latin America Cardiac POC Testing Devices and Kits Market Revenue By End User
14.3. Brazil
14.4. Argentina
14.5. Rest of Latin America
CHAPTER NO. 15 : MIDDLE EAST CARDIAC POC TESTING DEVICES AND KITS MARKET – COUNTRY ANALYSIS
15.1. Middle East Cardiac POC Testing Devices and Kits Market Overview By Country Segment
15.1.1. Middle East Cardiac POC Testing Devices and Kits Market Revenue Share By Region
15.2. Middle East
15.2.1. Middle East Cardiac POC Testing Devices and Kits Market Revenue By Country
15.2.2. Product
15.2.3. Middle East Cardiac POC Testing Devices and Kits Market Revenue By Product
15.2.4. End User
15.2.5. Middle East Cardiac POC Testing Devices and Kits Market Revenue By End User
15.3. GCC Countries
15.4. Israel
15.5. Turkey
15.6. Rest of Middle East
CHAPTER NO. 16 : AFRICA CARDIAC POC TESTING DEVICES AND KITS MARKET – COUNTRY ANALYSIS
16.1. Africa Cardiac POC Testing Devices and Kits Market Overview By Country Segment
16.1.1. Africa Cardiac POC Testing Devices and Kits Market Revenue Share By Region
16.2. Africa
16.2.1. Africa Cardiac POC Testing Devices and Kits Market Revenue By Country
16.2.2. Product
16.2.3. Africa Cardiac POC Testing Devices and Kits Market Revenue By Product
16.2.4. End User
16.2.5. Africa Cardiac POC Testing Devices and Kits Market Revenue By End User
16.3. South Africa
16.4. Egypt
16.5. Rest of Africa
CHAPTER NO. 17 : COMPANY PROFILES
17.1. Nexus Dx, Inc
17.1.2. Product Portfolio
17.1.3. Financial Overview
17.1.4. Recent Developments
17.1.5. Growth Strategy
17.1.6. SWOT Analysis
17.2. LifeSign LLC
17.3. Alfa Scientific Designs, Inc.
17.4. Spectral Diagnostics
17.5. Roche Diagnostics International Ltd.
17.6. American Screening Corporation, Inc.
17.7. Abbott
17.8. Siemens Ltd.
17.9. Other Key Players