Market Overview
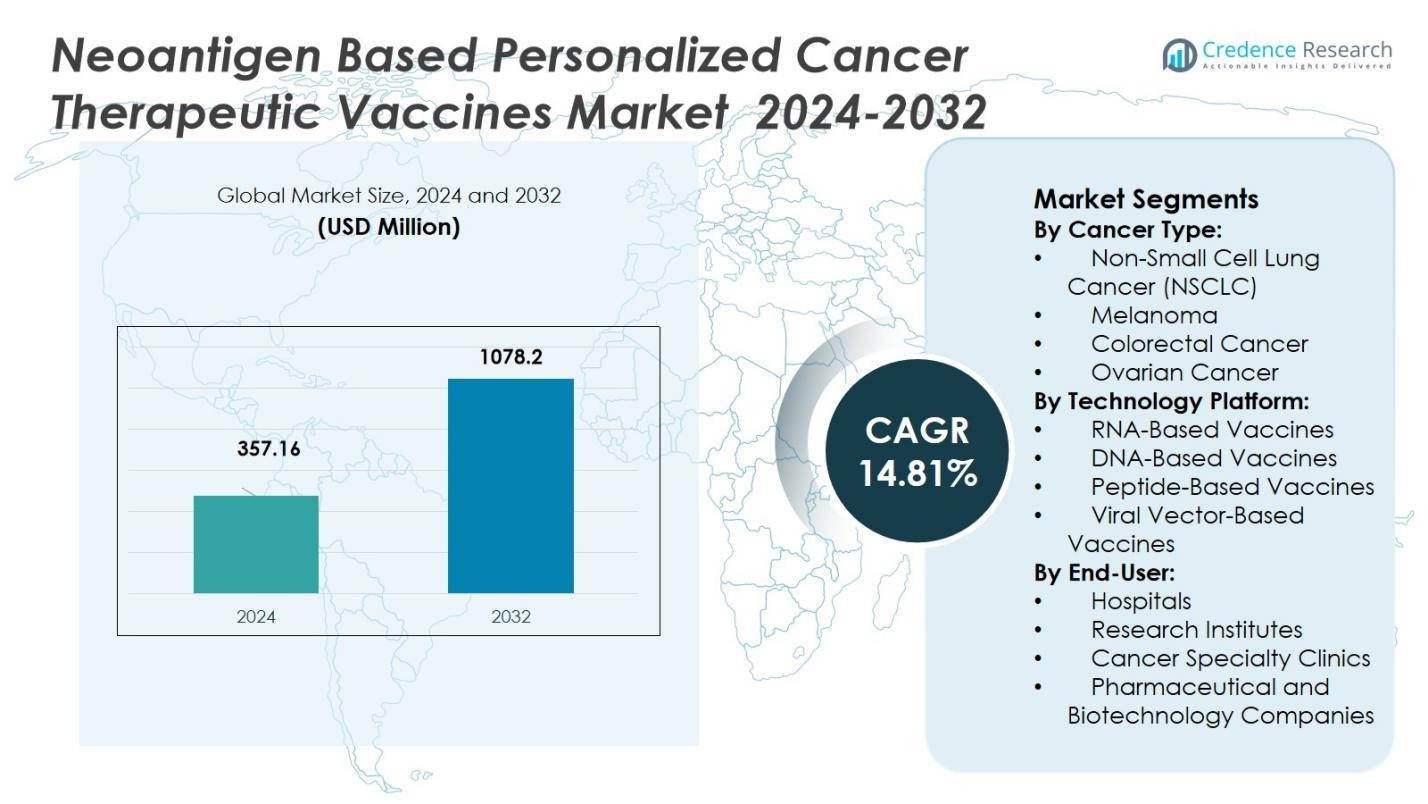
The Neoantigen-Based Personalized Cancer Therapeutic Vaccines Market was valued at USD 357.16 million in 2024 and is anticipated to reach USD 1078.2 million by 2032, growing at a CAGR of 14.81% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Neoantigen-Based Personalized Cancer Therapeutic Vaccines Market Size 2024 |
USD 357.16 Million |
| Neoantigen-Based Personalized Cancer Therapeutic Vaccines Market, CAGR |
14.81% |
| Neoantigen-Based Personalized Cancer Therapeutic Vaccines Market Size 2032 |
USD 1078.2 Million |
Neoantigen‑Based Personalized Cancer Therapeutic Vaccines market features prominent players including BioNTech SE, Moderna, Inc., CureVac AG, Gritstone Bio, Inc., Transgene S.A., Evaxion Biotech, Elicio Therapeutics, Agenus Inc., Imugene Ltd., and OSE Immunotherapeutics. North America emerges as the leading region with a market share of 45%, supported by its advanced biotechnology ecosystem, robust R&D infrastructure and early adoption of personalized immunotherapies. These companies leverage cutting‑edge mRNA, viral‑vector and peptide platforms to develop patient‑specific neoantigen vaccines. As these firms advance clinical pipelines and scale manufacturing capabilities, North America’s dominance is likely to strengthen, reinforcing its position as the primary driver of global market growth.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The Neoantigen-Based Personalized Cancer Therapeutic Vaccines Market was valued at USD 357.16 million in 2024 and is expected to reach USD 1078.2 million by 2032, growing at a CAGR of 14.81% during the forecast period.
- Increasing cancer prevalence, particularly in non-small cell lung cancer (NSCLC), melanoma, and colorectal cancer, is driving the market’s growth. Personalized treatments based on neoantigens offer potential for improved patient outcomes.
- The demand for personalized cancer treatments is accelerating due to advancements in mRNA technology, AI-driven neoantigen prediction, and the growing adoption of immunotherapies in oncology.
- Key market players, including BioNTech, Moderna, and CureVac, are leading the development of neoantigen vaccines using RNA-based, DNA-based, and peptide platforms, enhancing treatment efficacy.
- North America holds the largest market share of 45% in 2024, driven by strong healthcare infrastructure, while Asia-Pacific is the fastest-growing region, with increasing investments and rising cancer incidence.
 Market Segmentation Analysis:
Market Segmentation Analysis:
By Cancer Type:
The Neoantigen-Based Personalized Cancer Therapeutic Vaccines market is segmented by cancer type, with Non-Small Cell Lung Cancer (NSCLC) leading the segment, holding a dominant share of 40% in 2024. This is driven by the high prevalence of NSCLC and the significant unmet medical need for personalized treatments. Other key cancer types include melanoma, colorectal cancer, and ovarian cancer, each showing strong growth potential. The increasing number of clinical trials and FDA approvals for NSCLC vaccines are key drivers of this segment’s dominance in the market.
- For instance, the NEO-PV-01 personalized neoantigen vaccine combined with anti-PD-1 antibodies has shown safety and immune response efficacy in early-phase clinical trials for advanced NSCLC at institutions like the University of California and Massachusetts General Hospital.
By Technology Platform:
In terms of technology platforms, RNA-Based Vaccines hold the largest share of 45% in the Neoantigen-Based Personalized Cancer Therapeutic Vaccines market. This dominance is attributed to the success of mRNA platforms, demonstrated by the rapid development of COVID-19 vaccines, and their potential for personalized cancer immunotherapy. DNA-Based Vaccines follow with a share of 30%, while Peptide-Based and Viral Vector-Based Vaccines account for 15% and 10%, respectively. The flexibility and scalability of RNA vaccines continue to drive their market leadership, enabling faster development and production of personalized treatments.
- For instance, Pfizer-BioNTech and Moderna demonstrated the rapid development and large-scale deployment of COVID-19 mRNA vaccines, validating the platform’s speed and efficacy for personalized medicine applications.
By End-User:
The Neoantigen-Based Personalized Cancer Therapeutic Vaccines market is segmented by end-user, with hospitals leading the market with a share of 50% in 2024. Hospitals are the primary healthcare setting for personalized cancer vaccine treatments, driven by the growing demand for advanced oncology care and the integration of personalized therapies. Research institutes and cancer specialty clinics follow, with shares of 25% and 15%, respectively. Pharmaceutical and biotechnology companies hold a 10% share, contributing significantly through vaccine development and commercialization efforts in collaboration with healthcare providers.
Key Growth Drivers
Increasing Prevalence of Cancer
The rising global incidence of cancer, particularly non-small cell lung cancer (NSCLC), melanoma, and colorectal cancer, is a major growth driver for the Neoantigen-Based Personalized Cancer Therapeutic Vaccines market. With an aging population and lifestyle factors contributing to higher cancer rates, there is a growing demand for targeted, personalized treatments. Personalized cancer vaccines based on neoantigens offer hope for improving patient outcomes by boosting immune responses, thereby driving investment and innovation in this area. This surge in cancer cases is expected to sustain long-term market growth.
- For instance, Moderna and Merck reported in 2023 that their personalized mRNA cancer vaccine, mRNA-4157 (V940), combined with pembrolizumab, reduced the risk of melanoma recurrence or death by 44 percent in Phase 2 trials.
Advancements in mRNA Technology
The success of mRNA vaccines for COVID-19 has significantly accelerated the development of mRNA-based therapies in cancer treatment. The ability to customize vaccines to individual tumors using mRNA technology offers a promising avenue for personalized cancer immunotherapies. This advancement has led to increased confidence in the efficacy of neoantigen-based vaccines and generated substantial investments in research and clinical trials. The growing adoption of mRNA-based platforms for cancer treatment is expected to continue driving the market forward, particularly in the development of faster, more scalable treatments.
- For instance, BioNTech is advancing its BNT122 program, which delivers individualized mRNA vaccines targeting specific tumor neoantigens, currently being evaluated in Phase II trials for pancreatic cancer.
Government Support and Funding
Government initiatives and funding for cancer research and personalized medicine are pivotal drivers of growth in the Neoantigen-Based Personalized Cancer Therapeutic Vaccines market. Various regulatory bodies, such as the FDA and EMA, are increasingly prioritizing the development of personalized cancer treatments due to their potential to improve patient outcomes. This has led to favorable policies, including grants and incentives for clinical trials and innovations in vaccine technologies. Ongoing financial support ensures a conducive environment for accelerating the development and commercialization of personalized cancer vaccines.
Key Trends & Opportunities
Growing Demand for Personalized Medicine
Personalized medicine is increasingly becoming a key trend in oncology, and Neoantigen-Based Personalized Cancer Therapeutic Vaccines are at the forefront of this shift. The ability to tailor treatments based on an individual’s genetic profile and cancer mutations is seen as the next frontier in cancer care. This trend is gaining momentum as patients and healthcare providers demand more effective, targeted therapies that minimize side effects. Opportunities for growth lie in developing vaccines that can be easily customized for different cancer types and individual patients, driving future market expansion.
- For instance, Moderna’s mRNA-4157 (V940), developed in collaboration with Merck, demonstrated a 49% reduction in the risk of recurrence or death in melanoma patients when combined with pembrolizumab, according to Phase 2b clinical data published in The Lancet in 2023.
Integration of Artificial Intelligence (AI) in Vaccine Development
Artificial Intelligence (AI) is revolutionizing the development of personalized cancer vaccines by improving the identification of neoantigens. AI and machine learning algorithms can analyze vast datasets from genomic sequencing to predict the most effective neoantigens for individual patients. This integration enhances the accuracy of vaccine development, accelerates the discovery process, and reduces costs. The growing use of AI in vaccine design presents an exciting opportunity for companies to improve the precision and efficacy of personalized vaccines, offering competitive advantages in the market.
- For instance, AI algorithms have been instrumental in designing mRNA vaccines targeting the SARS-CoV-2 spike protein, significantly improving immunogenicity and accelerating development timelines from years to months.
Key Challenges
High Development Costs
One of the key challenges in the Neoantigen-Based Personalized Cancer Therapeutic Vaccines market is the high cost of development. Creating personalized vaccines requires sophisticated technology, extensive clinical trials, and substantial R&D investments. The process of sequencing patient tumors, identifying neoantigens, and developing tailored vaccines is resource-intensive. These high costs can limit the accessibility of personalized vaccines, particularly in regions with less healthcare funding. To overcome this challenge, companies must focus on reducing production costs while maintaining efficacy and safety, enabling broader market access.
Regulatory Hurdles and Approval Delays
Regulatory challenges remain a significant barrier to the widespread adoption of Neoantigen-Based Personalized Cancer Therapeutic Vaccines. The approval process for personalized vaccines is complex and lengthy, involving multiple stages of clinical testing to demonstrate safety and efficacy. Regulatory bodies must evaluate each treatment on a case-by-case basis, which can result in delays in getting products to market. Streamlining regulatory processes and ensuring clear guidelines for personalized treatments will be crucial in addressing these delays and ensuring timely access to these therapies for patients.
Regional Analysis
North America
North America leads the global neoantigen-based personalized cancer therapeutic vaccines market, accounting for a market share of 45% in 2024. The region’s dominance is driven by its robust healthcare infrastructure, advanced immunotherapy R&D ecosystem, and high adoption of next-generation sequencing and personalized medicine. Major biotech and pharmaceutical companies drive vaccine development and commercialization, supported by strong regulatory frameworks and substantial public-private investments. High cancer incidence rates, particularly in solid tumors such as lung and melanoma, further stimulate demand for neoantigen-targeted therapies, sustaining market growth in the region.
Europe
Europe holds a significant position in the neoantigen cancer vaccine market, with a market share of 30% in 2024. The region benefits from well-established oncology centers, integrated diagnostics, and increasing adoption of immunotherapy. Countries such as Germany, the UK, and France are leading in clinical trials and vaccine development. Healthcare payor systems and rising awareness of precision oncology further support the demand for personalized vaccines. European biotech firms and research institutions play a vital role in advancing neoantigen vaccine research, contributing to the region’s steady growth in the market.
Asia-Pacific
Asia-Pacific is the fastest-growing region for neoantigen cancer vaccines, with a projected market share of 15% in 2024. The region’s growth is driven by rising cancer incidence, expanding healthcare infrastructure, and growing investment in biotechnology. Countries like China, India, and Japan are increasingly involved in clinical research and regulatory approvals for personalized immunotherapies. Government initiatives to enhance biomanufacturing and growing public-private funding are expected to accelerate the adoption of neoantigen-based vaccines, positioning Asia-Pacific as a major contributor to the global market’s expansion in the coming years.
Latin America
Latin America holds a relatively small market share of 5% in the global neoantigen cancer vaccine market in 2024. However, the region is witnessing an upward trend in oncology care with increasing investments in advanced cancer therapies. Improvements in diagnostic capacities and rising awareness of personalized medicine are expected to gradually foster the growth of neoantigen-based vaccines. Collaborations between local research institutes and global biotech firms suggest that Latin America could experience a more substantial presence in the market as it progresses in the adoption of personalized cancer treatments.
Middle East & Africa
Middle East & Africa contribute a smaller share of 5% to the global neoantigen cancer vaccine market, with ongoing challenges related to healthcare infrastructure and limited access to advanced oncology treatments. However, the region is beginning to show growth potential through increasing government health investments, collaborations with global biotech companies, and improving diagnostic capabilities. As awareness of personalized cancer treatment rises and immunotherapy infrastructure improves, the region is expected to gradually increase its market share in the coming years, contributing to the global growth of neoantigen-based cancer vaccines.
Market Segmentations:
By Cancer Type:
- Non-Small Cell Lung Cancer (NSCLC)
- Melanoma
- Colorectal Cancer
- Ovarian Cancer
By Technology Platform:
- RNA-Based Vaccines
- DNA-Based Vaccines
- Peptide-Based Vaccines
- Viral Vector-Based Vaccines
By End-User:
- Hospitals
- Research Institutes
- Cancer Specialty Clinics
- Pharmaceutical and Biotechnology Companies
By Geography
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Competitive Landscape
Competitive landscape in the neoantigen‑based personalized cancer therapeutic vaccines market is shaped by leading players such as BioNTech SE, Moderna, CureVac AG, Gritstone Bio, Transgene S.A., Evaxion Biotech, Elicio Therapeutics, Agenus Inc., Nouscom, and others. These organizations are leveraging advanced mRNA, viral‑vector and peptide platforms to develop individualized cancer vaccines tailored to patient‑specific tumor mutations. The competitive intensity arises from rapid pipeline expansion across multiple cancer types and aggressive investments in genomic sequencing, bioinformatics and manufacturing infrastructure to enable scalable neoantigen vaccine production. Strategic partnerships between large pharmaceutical companies and biotech firms further accelerate R&D, while clinical trial successes and regulatory momentum enhance market credibility. As a result, companies with proven technical platforms, strong R&D capacity and flexible manufacturing protocols are best positioned to capture market share in this fast‑evolving space.
Key Player Analysis
- BioNTech SE
- Moderna, Inc.
- CureVac AG
- Gritstone Bio, Inc.
- Elicio Therapeutics
- Evaxion Biotech
- Agenus Inc.
- Transgene S.A.
- Imugene Ltd.
- OSE Immunotherapeutics SA
Recent Developments
- In March 2025, Everest Medicines dosed the first patient with its internally‑developed personalized mRNA cancer vaccine EVM16.
- In January 2025, myNEO Therapeutics entered a partnership with University of Liverpool to launch a Phase 1 clinical trial of a personalized therapeutic cancer vaccine targeting non‑small cell lung cancer.
- In June 2025, Transgene S.A. and its collaborator NEC reported durable 24‑month disease‑free survival and sustained T‑cell responses in patients treated with their individualized cancer vaccine TG4050.
- In 2025, Moderna, Inc. in partnership with Merck & Co. continued to advance their individualized neoantigen vaccine mRNA-4157/V940, now under Phase III evaluation across multiple tumor types.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Report Coverage
The research report offers an in-depth analysis based on Cancer Type, Technology Platform, End User and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The number of clinical trials for personalized neoantigen vaccines is expected to increase substantially, broadening the range of cancer types treated.
- Advances in bioinformatics, next-generation sequencing, and machine-learning–driven neoantigen prediction will improve antigen selection accuracy and speed, reducing time from tumor sampling to vaccine administration.
- Vaccine delivery technologies (e.g., lipid nanoparticles, dendritic-cell or liposomal platforms) will evolve to enhance immunogenicity and safety, enabling more efficient and patient-friendly treatment regimes.
- Combining neoantigen vaccines with immune-modulatory therapies (e.g., immune checkpoint inhibitors) will increase overall therapeutic efficacy and help overcome tumor immune-resistance mechanisms.
- Expansion into adjuvant and maintenance therapy applications (post-surgery or to prevent recurrence) will extend the use cases beyond advanced cancer, expanding the patient pool.
- Emerging regulatory frameworks and growing recognition of personalized immunotherapies will accelerate approval pathways and reduce time-to-market for new neoantigen vaccines.
- Increased collaborations between biotech firms and academic research centers worldwide will drive innovation, diversify geographic clinical trial distribution, and accelerate global adoption.
- Reduced manufacturing costs, driven by scale-up of mRNA and peptide-based vaccine production facilities, will improve affordability and increase access in emerging markets.
- Growing patient and physician awareness of precision oncology and personalized immunotherapy will increase market demand and acceptance of neoantigen-based treatments.
- Continued demonstration of long-lasting immune memory and durable responses in clinical trials will build confidence in neoantigen vaccines as a mainstream therapeutic option, driving uptake.

 Market Segmentation Analysis:
Market Segmentation Analysis:

