Market Overview
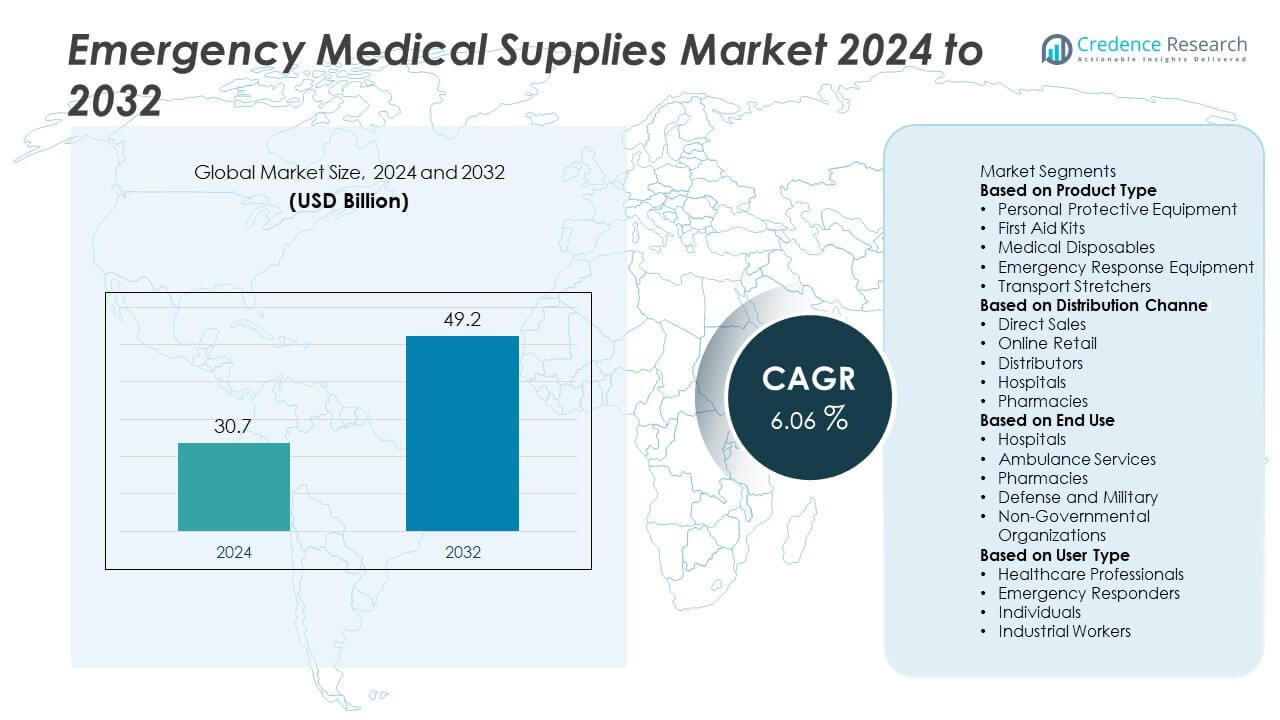
Emergency Medical Supplies Market was valued at USD 30.7 billion in 2024 and is expected to reach USD 49.2 billion by 2032, growing at a CAGR of 6.06% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Emergency Medical Supplies Market Size 2024 |
USD 30.7 Billion |
| Emergency Medical Supplies Market, CAGR |
6.06% |
| Emergency Medical Supplies Market Size 2032 |
USD 49.2 Billion |
The Emergency Medical Supplies Market grows with rising demand for rapid-response healthcare solutions, stricter safety regulations, and expanding healthcare infrastructure worldwide. Hospitals, trauma centers, and emergency units rely on advanced supplies such as protective equipment, wound care products, and diagnostic kits to manage critical situations
The Emergency Medical Supplies Market shows wide geographical presence, with North America leading due to advanced healthcare systems, high investments in preparedness programs, and strong adoption of portable emergency devices. Europe follows with strict regulatory standards, sustainability-focused initiatives, and well-established trauma care networks that secure consistent demand for protective equipment and diagnostic kits. Asia-Pacific demonstrates rapid growth supported by large-scale hospital expansions, government investments in disaster management, and strong local manufacturing capacity. Latin America and the Middle East & Africa contribute steadily, with rising healthcare reforms and growing awareness of emergency preparedness. Key players driving this market include Stryker, Fresenius Medical Care, Smith & Nephew, and Hillrom. It gains momentum from technological innovations by Fisher & Paykel Healthcare, Teleflex, and ConvaTec, which focus on portable, cost-efficient, and user-friendly solutions. Continuous investment in digital integration, partnerships, and advanced supply chains ensures a strong outlook for global emergency medical supplies.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- Emergency Medical Supplies Market was valued at USD 30.7 billion in 2024 and is projected to reach USD 49.2 billion by 2032, growing at a CAGR of 6.06% during the forecast period.
- Rising demand for rapid-response healthcare solutions, road safety needs, and government-backed preparedness programs drive consistent adoption of emergency supplies across hospitals and trauma centers.
- Market trends highlight strong growth in portable and smart devices, disposable protective equipment, and eco-friendly single-use products that enhance infection control and efficiency.
- Competitive landscape features leading companies such as Stryker, Fresenius Medical Care, Hillrom, Smith & Nephew, Fisher & Paykel Healthcare, Teleflex, and ConvaTec, focusing on innovation, partnerships, and product expansion.
- Market restraints include high production costs, raw material shortages, and strict regulatory standards that increase compliance burdens for manufacturers and slow product approvals.
- Regional analysis shows North America leading with strong infrastructure, Europe advancing with strict regulations and sustainability goals, Asia-Pacific expanding through large-scale healthcare growth, and Latin America and Middle East & Africa contributing with rising reforms and resource investments.
- Recycling initiatives, digital integration, and growing investment in portable devices create opportunities for suppliers to expand their presence and strengthen resilience against future crises.
Market Drivers
Growing Incidence of Accidents and Medical Emergencies Increasing Demand
The Emergency Medical Supplies Market grows with the rising incidence of road accidents, workplace injuries, and natural disasters. Hospitals, emergency response teams, and trauma centers require continuous access to life-saving equipment. It includes defibrillators, stretchers, wound care products, and diagnostic tools used in critical situations. Governments strengthen emergency preparedness systems, ensuring demand for advanced supplies. Rapid urbanization and increasing population density elevate the risk of mass-casualty events. It reinforces the urgent need for reliable and scalable medical supply chains. Consistent growth in emergency cases sustains the sector’s long-term relevance.
- For instance, Stryker’s LIFEPAK 15 defibrillator/monitor features biphasic defibrillation up to 360 joules and an integrated CPR metronome. These tools are designed to improve survival outcomes in cases of sudden cardiac arrest.
Expansion of Healthcare Infrastructure Strengthening Supply Utilization
The Emergency Medical Supplies Market benefits from the global expansion of healthcare infrastructure. Governments and private players invest heavily in hospitals, clinics, and specialized emergency units. It drives procurement of critical supplies, from monitoring devices to disposable medical products. Rising healthcare budgets support bulk purchasing programs and stockpiling initiatives. Growth in developing regions fuels new opportunities for suppliers entering underserved markets. It ensures greater accessibility to essential products across rural and urban populations. Infrastructure expansion directly strengthens demand for reliable emergency medical equipment.
- For instance, As of the end of 2024, Fresenius Medical Care operated a global network of roughly 3,700 dialysis clinics and provided care for more than 299,000 patients. Its standard hemodialysis machines, such as the 6008 CAREsystem and the 5008 CorDiax, can operate at blood flow rates of up to 600 ml/min.
Advancements in Technology Enhancing Product Efficiency
The Emergency Medical Supplies Market gains momentum from innovations that improve efficiency, portability, and reliability. Smart diagnostic devices, portable ventilators, and automated defibrillators enhance patient survival rates. It empowers first responders with tools designed for fast deployment and accurate intervention. Technology integration allows real-time monitoring and better coordination during emergencies. Rising R&D investments expand product portfolios to address diverse critical care needs. It supports the development of compact, multi-functional devices for field and hospital use. These advancements increase the overall effectiveness of emergency medical interventions.
Government Regulations and Preparedness Programs Supporting Market Growth
The Emergency Medical Supplies Market advances with policy-driven initiatives and preparedness programs. Governments enforce strict regulations requiring hospitals and emergency teams to maintain adequate stock. It creates stable demand and ensures compliance with safety standards. International organizations promote disaster preparedness strategies that encourage large-scale procurement. Defense and military sectors also drive consistent orders for field-ready medical supplies. It improves resilience against pandemics, mass emergencies, and cross-border crises. Public-private partnerships further enhance distribution efficiency and product innovation in the market.
Market Trends
Rising Demand for Portable and Point-of-Care Devices
The Emergency Medical Supplies Market shows strong demand for portable devices and point-of-care solutions. First responders and healthcare providers prioritize lightweight, mobile equipment for rapid deployment. It includes compact ventilators, portable monitors, and handheld diagnostic kits. Increased focus on quick treatment in ambulances and field settings drives innovation. Manufacturers design user-friendly devices that improve accuracy and reduce response times. It strengthens efficiency in critical care environments where speed determines patient outcomes. This trend accelerates adoption of advanced portable medical technologies worldwide.
- For instance, Philips’ HeartStart HS1 defibrillator weighs just 1.5 kilograms and can deliver shocks up to 150 joules, with an average time-to-shock of under 8 seconds, enabling rapid intervention by first responders.
Integration of Digital Health and Smart Monitoring Solutions
The Emergency Medical Supplies Market benefits from digital integration and smart monitoring systems. Connected devices enable real-time data sharing between field responders and hospitals. It supports early diagnosis and better coordination in emergencies. Wearable devices and wireless sensors expand applications beyond hospitals to remote settings. Artificial intelligence aids in predicting patient needs and optimizing treatment decisions. It enhances the functionality of emergency supplies by aligning them with telemedicine platforms. This trend transforms emergency care into a data-driven, responsive system.
- For instance, Fisher & Paykel Healthcare’s myAirvo 3 humidified high-flow system delivers flow rates of up to 70 liters per minute with integrated digital monitoring, enabling remote clinicians to track respiratory therapy performance in hospital and sub-acute facilities. The device has an integrated battery to facilitate patient transport within a facility.
Growing Emphasis on Disposable and Single-Use Products
The Emergency Medical Supplies Market records increased adoption of disposable and single-use products. Infection control remains a top priority for hospitals and emergency centers. It drives demand for sterile syringes, masks, gloves, and wound dressings. The trend gained momentum during global health crises, highlighting the importance of safety and hygiene. Manufacturers invest in eco-friendly disposables to address environmental concerns. It aligns with regulatory standards and institutional policies that mandate strict hygiene protocols. Rising demand for single-use products ensures consistent market growth.
Expansion of Public-Private Partnerships in Emergency Preparedness
The Emergency Medical Supplies Market experiences growth through collaboration between governments and private players. Public-private partnerships secure large-scale supply networks during emergencies. It ensures efficient distribution of life-saving equipment in crisis zones. Strategic alliances foster innovation and accelerate delivery timelines. Governments support procurement programs that guarantee stable demand for manufacturers. It enhances resilience against pandemics, disasters, and geopolitical conflicts. This trend strengthens the market’s ability to respond to large-scale health emergencies with efficiency.
Market Challenges Analysis
Supply Chain Disruptions and Raw Material Constraints Limiting Availability
The Emergency Medical Supplies Market faces significant challenges due to unstable supply chains and raw material shortages. Global crises, trade restrictions, and geopolitical tensions disrupt the flow of essential medical products. It creates delays in procurement of critical items such as ventilators, protective gear, and diagnostic kits. Limited raw material availability increases production costs and reduces manufacturing efficiency. Smaller suppliers struggle to compete with large corporations that secure long-term contracts. It forces governments and healthcare providers to rely on imports, raising concerns about accessibility during emergencies. Persistent disruptions weaken the ability to maintain adequate stock levels across regions.
High Costs and Stringent Regulations Affecting Market Scalability
The Emergency Medical Supplies Market is challenged by rising costs of advanced technologies and strict compliance requirements. Manufacturers face pressure to meet rigorous safety and quality standards imposed by global health authorities. It raises production expenses and slows product approvals, limiting innovation speed. Smaller firms encounter difficulties entering the market due to high capital demands. Regulatory variations across countries further complicate global distribution strategies. It creates barriers for uniform adoption of emergency supplies worldwide. Increasing costs and compliance burdens continue to challenge the scalability of the industry.
Market Opportunities
Advancement of Innovative and Portable Emergency Solutions Expanding Adoption
The Emergency Medical Supplies Market presents strong opportunities with the development of innovative and portable medical devices. Compact ventilators, wearable monitors, and AI-enabled diagnostic kits enhance efficiency during emergencies. It empowers first responders and healthcare providers with tools that improve patient survival rates. Growing investment in research supports the creation of multi-functional and easy-to-use devices. Demand for advanced trauma kits and mobile care units expands in both developed and emerging regions. It creates pathways for suppliers to strengthen their market presence. Increasing focus on innovation drives long-term opportunities across critical care and emergency response systems.
Rising Investments in Preparedness and Expansion of Global Healthcare Infrastructure
The Emergency Medical Supplies Market benefits from growing investments in healthcare preparedness and infrastructure development. Governments and organizations allocate resources to stockpile essential supplies for disaster and pandemic response. It enhances resilience and secures steady demand for critical equipment. Expansion of hospitals, clinics, and emergency care centers in developing regions widens opportunities for suppliers. Public-private partnerships encourage collaboration in procurement and distribution networks. It ensures faster availability of life-saving products in crisis situations. Strong infrastructure growth and preparedness programs strengthen the industry’s future expansion.
Market Segmentation Analysis:
By Product Type
The Emergency Medical Supplies Market is segmented by product type into wound care, personal protective equipment, cardiac care, diagnostic supplies, and others. Wound care products, including bandages, dressings, and hemostatic agents, remain widely used in hospitals and trauma centers. It ensures immediate care during accidents and emergencies. Personal protective equipment such as gloves, masks, and gowns saw accelerated demand during global health crises and continues to hold strong relevance. Cardiac care devices, including defibrillators and emergency kits, are essential for critical intervention. Diagnostic supplies, including portable test kits, support rapid assessments during emergencies. Each product segment plays a vital role in addressing diverse healthcare needs across emergency settings.
- For instance, Smith & Nephew’s ALLEVYN LIFE dressing offers a highly absorbent, five-layer design with a lock-away core that can be worn for up to 7 days, providing effective fluid management for a wide range of acute and chronic wounds, including surgical wounds, diabetic foot ulcers, and pressure injuries.
By Distribution Channel
The Emergency Medical Supplies Market is further segmented by distribution channel into hospital pharmacies, retail pharmacies, online platforms, and others. Hospital pharmacies dominate distribution due to their direct role in serving emergency departments and intensive care units. It enables timely access to critical products in high-pressure environments. Retail pharmacies provide wider accessibility to consumers for basic supplies, first aid kits, and protective equipment. Online platforms are expanding rapidly, offering convenience and broad product availability with quick delivery. It strengthens supply access across urban and rural areas, especially during crises. Other distribution channels, including specialized suppliers, support bulk orders for institutions and emergency response agencies.
- For instance, Walgreens Boots Alliance operates more than 12,500 retail pharmacy locations globally, each stocking emergency first aid kits and protective supplies to serve millions of customers annually with rapid access to essential medical products.
By End Use
The Emergency Medical Supplies Market includes end uses such as hospitals, trauma centers, ambulatory surgical centers, and others. Hospitals remain the largest consumers due to their comprehensive emergency care infrastructure and high patient inflow. It drives significant demand for advanced devices, disposable products, and life-saving kits. Trauma centers focus on rapid interventions for accident-related injuries, fueling steady use of wound care and diagnostic supplies. Ambulatory surgical centers create growing demand for cost-efficient and portable products to support outpatient emergencies. Other end users include defense, disaster relief agencies, and home healthcare providers. It ensures broad application of supplies across diverse emergency care scenarios.
Segments:
Based on Product Type
- Personal Protective Equipment
- First Aid Kits
- Medical Disposables
- Emergency Response Equipment
- Transport Stretchers
Based on Distribution Channel
- Direct Sales
- Online Retail
- Distributors
- Hospitals
- Pharmacies
Based on End Use
- Hospitals
- Ambulance Services
- Pharmacies
- Defense and Military
- Non-Governmental Organizations
Based on User Type
- Healthcare Professionals
- Emergency Responders
- Individuals
- Industrial Workers
Based on the Geography:
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis
North America
North America accounts for a 32.4% share of the Emergency Medical Supplies Market, driven by advanced healthcare infrastructure, high healthcare spending, and strong emergency preparedness programs. The region benefits from a large number of hospitals, trauma centers, and ambulatory surgical centers that demand consistent supplies of protective gear, wound care products, and diagnostic kits. It is further supported by government initiatives such as the U.S. Strategic National Stockpile, which ensures bulk procurement and distribution of critical supplies during crises. The rising frequency of road accidents, chronic conditions, and natural disasters in the United States and Canada continues to elevate demand. Investment in smart and portable medical technologies, coupled with digital health integration, further strengthens the region’s leadership position. Continuous innovation by local and global manufacturers enhances availability of high-quality emergency medical supplies across the market.
Europe
Europe holds a 28.1% share of the Emergency Medical Supplies Market, supported by strict regulatory frameworks, advanced medical facilities, and strong focus on safety standards. The European Union mandates stringent compliance for hospitals and emergency centers to maintain adequate levels of supplies. It ensures consistent demand across wound care, personal protective equipment, and cardiac emergency devices. Countries such as Germany, the UK, and France lead adoption, while Eastern Europe shows gradual growth with increasing healthcare investments. Strong government support for emergency preparedness programs and partnerships with private suppliers expand the reach of essential products. It is further reinforced by regional initiatives to improve disaster resilience, pandemic response, and cross-border supply chain integration. Continuous innovation in eco-friendly and disposable emergency supplies aligns with Europe’s sustainability goals, keeping the region highly competitive.
Asia-Pacific
Asia-Pacific dominates with a 30.7% share of the Emergency Medical Supplies Market, reflecting its large population base, rapid urbanization, and growing investments in healthcare infrastructure. China, Japan, India, and South Korea represent key growth drivers with expanding hospitals and rising awareness of emergency preparedness. It benefits from increasing government funding for disaster management and rising healthcare access in rural areas. Strong demand for protective equipment and diagnostic supplies has accelerated since recent health crises, creating long-term growth momentum. Local manufacturers scale production at competitive costs, making the region a global hub for supply. It also sees growing adoption of portable and digital emergency devices to meet rising healthcare needs. Asia-Pacific’s leadership is strengthened by both strong domestic consumption and export capacity for emergency medical products.
Latin America
Latin America captures a 5.4% share of the Emergency Medical Supplies Market, supported by growing investment in healthcare infrastructure and expanding demand for protective equipment. Brazil, Argentina, and Chile lead adoption due to higher healthcare spending and government-led initiatives to improve emergency services. It benefits from regional stockpiling programs and rising consumer awareness of personal medical preparedness. Limited availability of advanced technologies remains a barrier, but expanding partnerships with global suppliers address these gaps. Trauma care and accident response represent key areas of demand due to increasing road accidents and occupational injuries. It positions the region as a steadily growing market with untapped potential. Rising focus on disaster preparedness and emergency care reforms supports future expansion.
Middle East & Africa
The Middle East & Africa region holds a 3.4% share of the Emergency Medical Supplies Market, reflecting early-stage adoption and growing investments in healthcare systems. Gulf nations, including the UAE and Saudi Arabia, drive growth with large-scale investments in emergency infrastructure and preparedness initiatives. It is further supported by increasing government spending on healthcare modernization and pandemic response measures. In Africa, countries such as South Africa and Nigeria see rising demand for wound care and protective equipment due to growing accident cases and infectious disease outbreaks. International partnerships and aid programs supply critical products in regions with limited infrastructure. It creates opportunities for expansion as governments prioritize healthcare resilience. The region is expected to gain importance in the global market as both demand and local production capabilities expand gradually.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- ConvaTec
- Hillrom
- Bard
- Teleflex
- Fresenius Medical Care
- Smith & Nephew
- Halyard Health
- MediWare Information Systems
- Fisher & Paykel Healthcare
- Stryker
Competitive Analysis
The competitive landscape of the Emergency Medical Supplies Market is defined by leading players including Stryker, Fresenius Medical Care, Hillrom, Smith & Nephew, Fisher & Paykel Healthcare, Teleflex, ConvaTec, Halyard Health, MediWare Information Systems, and R. Bard. These companies maintain strong market positioning through diverse product portfolios, global distribution networks, and continuous innovation in emergency healthcare solutions. The industry is characterized by steady demand for wound care, protective equipment, cardiac emergency devices, and portable diagnostic tools, driving suppliers to invest in research and development. It encourages advancements in disposable and eco-friendly products, digital health integration, and compact, user-friendly devices suited for both hospitals and field use. Strategic collaborations with healthcare providers, government agencies, and distributors further strengthen their presence across developed and emerging markets. The competitive focus remains on enhancing efficiency, ensuring regulatory compliance, and addressing cost challenges while maintaining high standards of patient safety. Together, these strategies secure the long-term resilience and growth of the market.
Recent Developments
- In July 2025, Teleflex completed its acquisition of BIOTRONIK’s Vascular Intervention business, adding a broad suite of vascular intervention devices—including drug-coated balloons and ultrathin drug-eluting stents—to its portfolio.
- In March 2025, ConvaTec launched two educational programs in partnership with the WOCN Society, aiming to train over 750 new healthcare professionals in advanced ostomy care via online Advanced Ostomy Care and Ostomy Care Associates programs.
- In March 2025, Stryker introduced the Artix™ Thrombectomy System (via Inari Medical), engineered for peripheral arterial clots. It features an 8 Fr low-profile sheath (available in 65 cm and 90 cm), enabling both aspiration and mechanical clot removal in a single procedure.
- In November 2024, Fisher & Paykel Healthcare introduced the F&P Nova Micro™, its smallest and lightest nasal pillows mask yet, now available in the U.S., for obstructive sleep apnea therapy.
Report Coverage
The research report offers an in-depth analysis based on Product Type, Distribution Channel, End Use, User Type and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- Rising healthcare emergencies will continue to increase global demand for advanced medical supplies.
- Portable and smart emergency devices will gain wider adoption in hospitals and field settings.
- Disposable and eco-friendly single-use products will become more prominent to support infection control.
- Governments will strengthen preparedness programs and stockpiling initiatives to ensure supply stability.
- Digital health integration will enhance monitoring, diagnostics, and coordination during emergency care.
- Investment in healthcare infrastructure across developing regions will create new opportunities for suppliers.
- Strategic collaborations between manufacturers and public agencies will expand market reach.
- Supply chain resilience and localization will become critical to overcome disruption risks.
- Innovation in multi-functional and user-friendly devices will define competitive differentiation.
- Global focus on sustainability and regulatory compliance will shape long-term industry practices.




