Market Overview
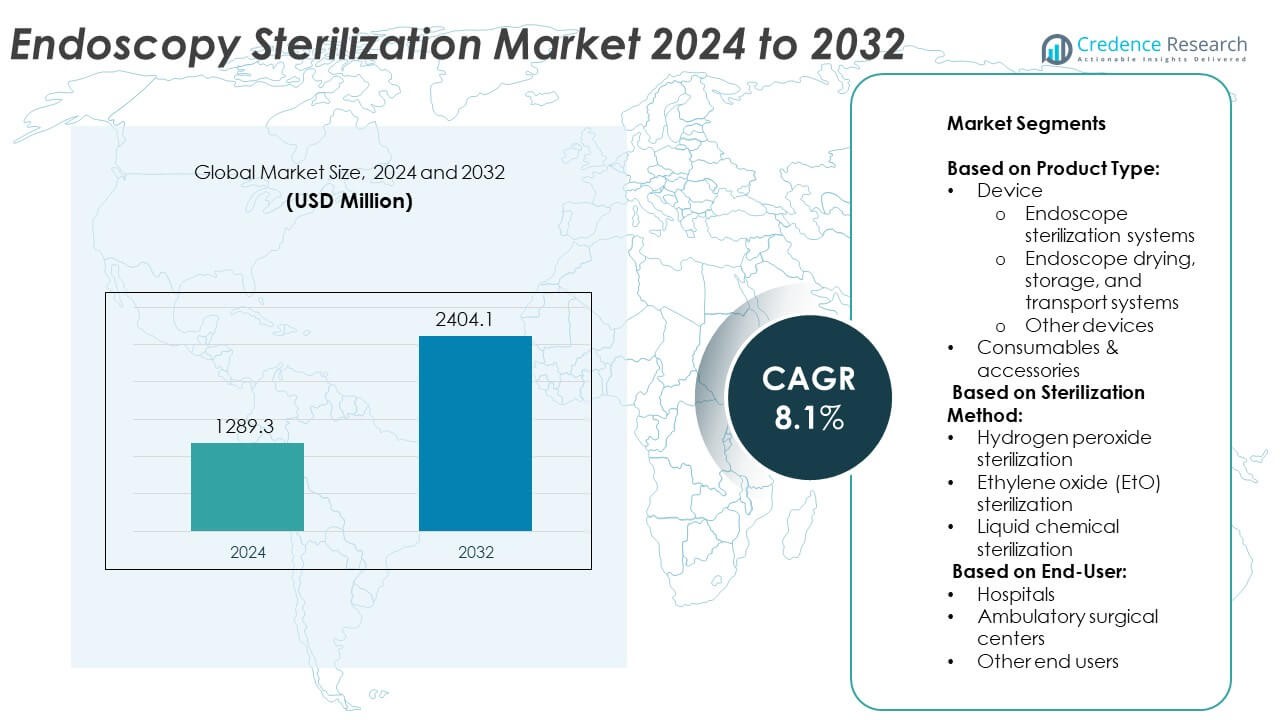
The Endoscopy Sterilization Market size was valued at USD 1289.3 million in 2024 and is anticipated to reach USD 2404.1 million by 2032, at a CAGR of 8.1% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2024 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Endoscopy Sterilization Market Size 2024 |
USD 1289.3 Million |
| Endoscopy Sterilization Market, CAGR |
8.1% |
| Endoscopy Sterilization Market Size 2032 |
USD 2404.1 Million |
The Endoscopy Sterilization market is driven by the increasing demand for effective infection control, rising volumes of minimally invasive procedures, and strict regulatory guidelines for reprocessing medical devices. Healthcare facilities prioritize advanced sterilization systems that ensure patient safety and operational efficiency. Technological advancements, including automated systems, rapid-cycle sterilization, and smart monitoring, are reshaping market dynamics. Sustainability trends encourage the adoption of energy-efficient and environmentally friendly sterilization methods. Expanding healthcare infrastructure in emerging regions further boosts demand, while continuous innovation in sterilization techniques and equipment design supports higher throughput, consistent performance, and compliance with global safety and quality standards.
The Endoscopy Sterilization market spans North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa, with growth influenced by advanced healthcare systems in developed regions and rapid infrastructure expansion in emerging economies. North America and Europe lead in adopting automated and high-precision sterilization technologies, while Asia Pacific sees rising demand driven by increasing endoscopic procedures. Key players such as STERIS, Getinge, and Tuttnauer play a significant role through their innovative product portfolios, global distribution capabilities, and strong focus on regulatory compliance. These companies emphasize reliability, efficiency, and sustainability in their sterilization solutions to meet evolving healthcare needs.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The Endoscopy Sterilization market was valued at USD 1289.3 million in 2024 and is projected to reach USD 2404.1 million by 2032, registering a CAGR of 8.1% during the forecast period.
- Rising adoption of advanced sterilization systems in hospitals and surgical centers is driven by the need to prevent cross-contamination and meet stringent infection control regulations.
- Increasing integration of automation, IoT-enabled monitoring, and rapid-cycle sterilization technologies enhances process efficiency, accuracy, and operational safety.
- The competitive landscape features key players such as STERIS, Getinge, Tuttnauer, and Matachana, focusing on innovation, global expansion, and compliance with regulatory standards.
- High initial investment costs and technical integration challenges in existing healthcare setups act as restraints for smaller facilities with limited budgets.
- North America and Europe lead in technology adoption due to established healthcare systems, while Asia Pacific is experiencing rapid growth fueled by infrastructure expansion and rising procedure volumes.
- The market benefits from growing opportunities in emerging economies, where government healthcare investments and awareness of infection prevention are boosting the demand for efficient, compliant sterilization solutions.
Market Drivers
Growing Emphasis on Patient Safety and Infection Control
The Endoscopy Sterilization benefits from stringent healthcare protocols aimed at preventing cross-contamination during endoscopic procedures. Hospitals and clinics prioritize advanced sterilization systems that integrate disconnect switch mechanisms for operational safety. Regulatory bodies enforce strict sterilization compliance, pushing facilities to adopt reliable solutions. The demand for equipment that minimizes human error during high-level disinfection continues to increase. Integration of safety switches ensures that sterilization cycles cannot be interrupted accidentally, maintaining process integrity. This focus on safety drives continuous investment in upgraded sterilization infrastructure.
- For instance, Getinge’s SMART system enhances sterilization reliability and efficiency by reducing cycle times by 15-20%. This improvement is achieved through advanced process control, leading to more consistent and reliable sterilization outcomes.
Advancements in Automated Sterilization Technologies
Automation in sterilization processes supports efficiency and consistency in endoscopy reprocessing. The Endoscopy Sterilization market benefits from systems that incorporate automated control features with precise cycle monitoring. It enables facilities to reduce manual intervention and ensure compliance with validated protocols. Manufacturers are designing equipment that links sterilization parameters to disconnect switch operations for enhanced control. These innovations reduce the risk of incomplete sterilization, improving patient outcomes. The shift toward automation strengthens the adoption of advanced sterilization solutions across high-volume healthcare facilities.
- For instance, AI-based predictive maintenance reduced equipment repair costs by approximately 2 million dollars annually in UK healthcare facilities while improving compliance.
Rising Procedure Volumes and Endoscopy Utilization
Growing demand for minimally invasive diagnostics and treatments fuels the need for effective sterilization equipment. The Endoscopy Sterilization market aligns with this demand by ensuring reliable power control during reprocessing. It supports uninterrupted sterilization cycles in settings where procedure volumes are increasing steadily. Healthcare providers seek equipment capable of handling multiple reprocessing cycles per day without performance degradation. Disconnect switches help safeguard the process against electrical malfunctions during peak operational loads. This reliability contributes to sustaining the efficiency of busy endoscopy units.
Regulatory Compliance and Standardization Requirements
Global health authorities set clear guidelines for sterilization of reusable medical devices, including endoscopes. The Endoscopy Sterilization market aligns with these standards by incorporating safety interlocks that ensure process completion. It assists facilities in meeting certification requirements by preventing premature equipment shutdowns. Standardized safety protocols drive consistent equipment performance and user adherence to operating instructions. The integration of disconnect switches into sterilization systems also facilitates easier inspection and validation. Compliance with these requirements supports long-term trust in equipment reliability and safety.
Market Trends
Integration of Smart Monitoring and Control Systems
The Endoscopy Sterilization market is experiencing a shift toward smart-enabled solutions that enhance process visibility and safety. Manufacturers are embedding sensors and IoT capabilities into disconnect switches to track operational parameters in real time. It enables maintenance teams to detect faults early and prevent costly equipment downtime. Automated alerts and data logging functions support compliance with sterilization protocols. Facilities benefit from improved operational oversight and reduced manual monitoring requirements. This trend aligns with the broader movement toward digitalization in healthcare infrastructure.
- For instance, STERIS’ SONICU environmental monitoring system supports over 500 sensors per hub and sends real-time alerts via text, email, push notifications, or phone to ensure consistent sterilization environments.
Focus on Compact and Modular Equipment Designs
Healthcare facilities are seeking sterilization systems that optimize space without compromising performance. The Endoscopy Sterilization supports this demand through compact, modular designs that integrate seamlessly into reprocessing areas. It allows for easier installation and maintenance in facilities with limited space. Modular components enable quick replacements, reducing service interruptions. This design approach also supports scalability as healthcare centers expand endoscopy services. Demand for space-efficient solutions continues to grow in both developed and emerging healthcare markets.
- For instance, Tuttnauer’s hospital-range autoclaves offer chamber sizes from 310 to 1,010 liters, designed for reliable processing within spatial constraints.
Adoption of Energy-Efficient and Sustainable Solutions
Sustainability objectives are influencing the design of sterilization systems and supporting components. The Endoscopy Sterilization market is aligning with this trend by incorporating low-energy consumption mechanisms and durable materials. It reduces operational costs while meeting environmental regulations. Manufacturers are focusing on switches with extended service life to minimize waste generation. Energy-efficient systems appeal to healthcare providers aiming to lower carbon footprints without sacrificing performance. This focus on sustainability strengthens the market’s long-term growth prospects.
Customization to Meet Diverse Clinical Requirements
Different healthcare facilities have varied sterilization needs based on procedure volume and equipment type. The Endoscopy Sterilization market addresses these needs with customizable features such as adjustable voltage ratings and tailored safety interlocks. It ensures compatibility with a range of sterilization systems, from high-capacity hospital units to smaller clinic setups. Customization supports enhanced workflow integration and operational efficiency. Manufacturers offering adaptable solutions gain a competitive edge by meeting specific client requirements. This trend encourages continuous innovation in product design and functionality.
Market Challenges Analysis
High Initial Investment and Maintenance Costs
The Endoscopy Sterilization market faces challenges due to the significant capital required for advanced sterilization equipment and integrated safety systems. Smaller healthcare facilities often struggle to allocate budgets for high-quality disconnect switches that meet regulatory standards. It becomes more complex when factoring in ongoing maintenance and periodic safety inspections. Specialized components may require certified technicians, increasing service expenses. This cost barrier can delay technology upgrades in cost-sensitive markets. The financial constraint impacts adoption rates, particularly in regions with limited healthcare funding.
Technical Integration and Compatibility Issues
Ensuring seamless integration of disconnect switches with diverse sterilization systems remains a critical challenge. The Endoscopy Sterilization market must address variations in equipment design, voltage specifications, and operational workflows across different healthcare facilities. It requires manufacturers to develop adaptable solutions without compromising safety and performance. Incompatibility can lead to installation delays, operational inefficiencies, and higher customization costs. Facilities operating older equipment face additional hurdles in retrofitting disconnect switches into existing setups. These challenges highlight the need for versatile designs and standardized technical specifications.
Market Opportunities
Expansion in Emerging Healthcare Markets
The Endoscopy Sterilization market has significant potential in developing regions where healthcare infrastructure is expanding rapidly. Rising investments in hospital construction and diagnostic centers create demand for advanced sterilization solutions. It provides an opportunity for manufacturers to introduce cost-effective and adaptable disconnect switch systems. Growing awareness of infection control practices among healthcare providers supports faster adoption. Suppliers who offer training and after-sales support can strengthen their market presence in these regions. This expansion potential is reinforced by government initiatives to improve patient safety standards.
Growth in Demand for Technologically Advanced Solutions
Increasing preference for automated and smart-enabled sterilization equipment opens avenues for innovation. The Endoscopy Sterilization market can leverage this trend by integrating advanced monitoring, fault detection, and remote control capabilities. It allows healthcare facilities to improve operational efficiency while ensuring strict compliance with sterilization protocols. Customizable features can cater to different facility sizes and procedure volumes, broadening the addressable market. Manufacturers focusing on energy efficiency and long product life can appeal to sustainability-conscious buyers. These opportunities create a pathway for long-term competitive advantage.
Market Segmentation Analysis:
By Product Type:
The Endoscopy Sterilization market is segmented into devices and consumables & accessories. Devices include endoscope sterilization systems, endoscope drying, storage, and transport systems, and other related equipment. It plays a critical role in ensuring that sterilization processes operate without interruption and meet safety standards. Endoscope sterilization systems dominate due to their essential function in infection control, while drying and storage systems support moisture removal and contamination prevention. Other devices include auxiliary units that complement primary sterilization systems. Consumables and accessories encompass sterilant solutions, filters, and protective covers, which are in continuous demand to maintain operational efficiency.
- For instance, STERIS’s Reliance Endoscope Processing System performs a high‑level disinfection cycle that includes a 6‑minute exposure, followed by HEPA‑filtered air purge, and an automatic 0.2 µm filter integrity check to alert users if a failure occurs.
By Sterilization:
The market includes hydrogen peroxide sterilization, ethylene oxide (EtO) sterilization, and liquid chemical sterilization methods. Hydrogen peroxide sterilization is gaining preference for its shorter cycle times and compatibility with delicate endoscopes. It integrates well with modern disconnect switch systems that require precise power control to maintain sterilization parameters. EtO sterilization remains relevant for complex instruments but faces limitations due to longer aeration times and regulatory scrutiny. Liquid chemical sterilization offers flexibility for temperature-sensitive devices and supports high-level disinfection needs. Each method requires reliable safety controls to ensure uninterrupted operation and compliance with infection prevention protocols.
- For instance, Tuttnauer’s PlazMax low‑temperature hydrogen peroxide plasma sterilizer offers an “endoscope cycle” completing in 37 minutes within a 109‑liter chamber and tracks cycle data via R.PC.R. software for remote monitoring.
By End-User:
Hospitals represent the largest end-user segment, driven by high procedure volumes and strict regulatory requirements for sterilization safety. The Endoscopy Sterilization market supports hospital needs through systems designed for continuous, high-capacity operation. It also serves ambulatory surgical centers, which require compact, efficient sterilization solutions to manage diverse procedure schedules. Other end users, including diagnostic clinics and specialty centers, adopt these systems to enhance infection prevention measures. Demand across all end-user categories is reinforced by the need for standardized safety features, seamless integration, and reliable operational performance. This segmentation reflects the broad applicability of disconnect switch systems in safeguarding endoscopy reprocessing workflows.
Segments:
Based on Product Type:
- Endoscope sterilization systems
- Endoscope drying, storage, and transport systems
- Other devices
- Consumables & accessories
Based on Sterilization Method:
- Hydrogen peroxide sterilization
- Ethylene oxide (EtO) sterilization
- Liquid chemical sterilization
Based on End-User:
- Hospitals
- Ambulatory surgical centers
- Other end users
Based on the Geography:
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis
North America
North America holds 38% of the Endoscopy Sterilization market, supported by advanced healthcare infrastructure and high adoption rates of automated sterilization systems. Hospitals and surgical centers in the United States and Canada prioritize patient safety and compliance with stringent infection control regulations. It benefits from strong demand for equipment that integrates disconnect switch mechanisms to enhance operational reliability. The region’s leadership in adopting smart-enabled sterilization technologies drives continuous investment in product upgrades. Reimbursement policies and well-established endoscopy practices further strengthen market penetration. The presence of leading manufacturers and distributors ensures wide availability and faster adoption of innovative sterilization solutions.
Europe
Europe accounts for 29% of the market, driven by strict regulatory frameworks and a focus on sustainable healthcare solutions. Facilities across Germany, the United Kingdom, France, and other EU countries adopt sterilization equipment that meets rigorous operational and safety standards. The Endoscopy Sterilization market in this region benefits from growing adoption of energy-efficient systems that reduce environmental impact. It also gains momentum from widespread implementation of minimally invasive procedures, which increases the need for reliable reprocessing equipment. Government-led healthcare investments and procurement initiatives support demand for both new installations and upgrades. The region’s mature market environment fosters consistent product innovation and high user training standards.
Asia Pacific
Asia Pacific holds 21% of the market, supported by rapid healthcare infrastructure expansion in countries such as China, Japan, India, and Australia. Rising awareness of infection prevention protocols encourages hospitals and diagnostic centers to invest in advanced sterilization equipment. The Endoscopy Sterilization market benefits from the growing number of endoscopic procedures in both public and private facilities. It also gains traction from government healthcare modernization programs and private sector investments in surgical centers. Domestic manufacturing capabilities in China and Japan are improving product accessibility and reducing costs. Increasing medical tourism in countries like Thailand and India further fuels demand for efficient sterilization systems.
Latin America
Latin America accounts for 7% of the market, with growth concentrated in Brazil, Mexico, and Argentina. Hospitals and clinics are increasingly adopting standardized sterilization processes to align with international safety requirements. The Endoscopy Sterilization market in the region benefits from gradual upgrades to replace outdated sterilization equipment. It faces challenges related to budget constraints in public healthcare systems, but private hospitals are driving adoption through investment in advanced technologies. Training programs and distributor partnerships are improving equipment accessibility and operational knowledge. Rising demand for minimally invasive surgeries continues to create opportunities for modern reprocessing solutions.
Middle East & Africa
The Middle East & Africa hold 5% of the market, with adoption concentrated in Gulf Cooperation Council (GCC) countries and South Africa. Investment in modern hospital facilities and specialized surgical centers drives demand for advanced sterilization systems with integrated safety controls. The Endoscopy Sterilization market benefits from ongoing healthcare diversification initiatives aimed at improving patient safety standards. It gains further momentum from medical infrastructure projects in countries such as the UAE, Saudi Arabia, and Qatar. Limited local manufacturing capacity makes imports the primary supply source, increasing reliance on global suppliers. Gradual expansion of private healthcare services in African nations also contributes to market growth.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
Competitive Analysis
The leading players in the Endoscopy Sterilization market include STERIS, Getinge, Tuttnauer, Matachana, Steelco, HUMAN MEDITEK, Stryker Corporation, MMM Group, Andersen Products, ASP, and AURORA. These companies maintain a strong competitive position through advanced product portfolios, global distribution networks, and consistent investment in research and development. They focus on delivering sterilization systems that ensure high operational efficiency, compliance with stringent infection control standards, and integration with automated reprocessing workflows. Product differentiation is achieved through technological innovations such as rapid-cycle sterilization, energy-efficient designs, and smart monitoring features that enhance safety and traceability. Strategic collaborations, acquisitions, and expansion into emerging healthcare markets strengthen their global presence and customer base. Continuous training programs and after-sales service further reinforce brand loyalty, while sustainability-focused designs align with healthcare providers’ environmental objectives. By addressing diverse clinical needs and regulatory requirements, these companies sustain a competitive edge in both mature and developing markets.
Recent Developments
- In 2024, Steelco introduced new sterilization technology for endoscopy by launching a new range of low-temperature sterilizers and developing reprocessing solutions specifically designed for instrumentation, including those for Da Vinci robotic surgery systems. This reflects Steelco’s ongoing technological innovation in instrument reprocessing with a focus on enhanced efficiency and safety in sterilization processes.
- In 2024, Matachana continues to innovate in low temperature sterilization technology, notably with their LF130 sterilizer model using a formaldehyde-based low temperature sterilization process. The sterile solution contains 2% formaldehyde, and the device supports delicate, thermosensitive medical instruments including endoscopes.
- In June 2024, Getinge launched the Poladus 150, a low-temperature sterilizer designed specifically for heat-sensitive endoscopes. This launch expands Getinge’s presence in the specialized sterilization device market. The Poladus 150 utilizes vaporized hydrogen peroxide (VH2O2) and operates at a gentle temperature of up to 55°C, making it suitable for various instruments used in endoscopy and surgical procedures.
Market Concentration & Characteristics
The Endoscopy Sterilization market displays a moderately consolidated structure, with a few global manufacturers holding significant influence due to their advanced technological capabilities, strong brand presence, and extensive distribution networks. It is characterized by high entry barriers driven by stringent regulatory requirements, substantial capital investments, and the need for specialized expertise in sterilization technologies. Leading companies differentiate their offerings through innovation in automation, energy efficiency, and smart monitoring systems that enhance operational safety and compliance. The market emphasizes reliability, consistent performance, and compatibility with diverse endoscopic equipment, reflecting the critical role sterilization plays in infection control. Product development cycles are often shaped by evolving healthcare regulations and hospital procurement standards, driving continuous improvements in safety features and environmental sustainability. Competition remains focused on delivering solutions that meet varying facility sizes, procedure volumes, and regional regulatory demands while maintaining cost efficiency and long-term operational value.
Report Coverage
The research report offers an in-depth analysis based on Product Type, Sterilization Method, End-User and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- Growing demand for automated sterilization systems with integrated safety controls will drive adoption.
- Increasing use of IoT-enabled monitoring will improve process efficiency and traceability.
- Rising procedure volumes in minimally invasive surgeries will boost equipment utilization rates.
- Expansion of healthcare infrastructure in emerging economies will create new market opportunities.
- Development of energy-efficient sterilization technologies will align with sustainability goals.
- Customizable solutions will cater to diverse clinical needs and facility sizes.
- Integration of rapid-cycle sterilization methods will enhance turnaround times in high-volume centers.
- Compliance with evolving global regulatory standards will influence product design and innovation.
- Strategic partnerships and mergers will strengthen global distribution and service networks.
- Continuous investment in research and development will foster competitive differentiation.




