| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| France Electrophysiology Devices Market Size 2024 |
USD 494.60 million |
| France Electrophysiology Devices Market, CAGR |
14.04% |
| France Electrophysiology Devices Market Size 2032 |
USD 1,414.53 million |
Market Overview
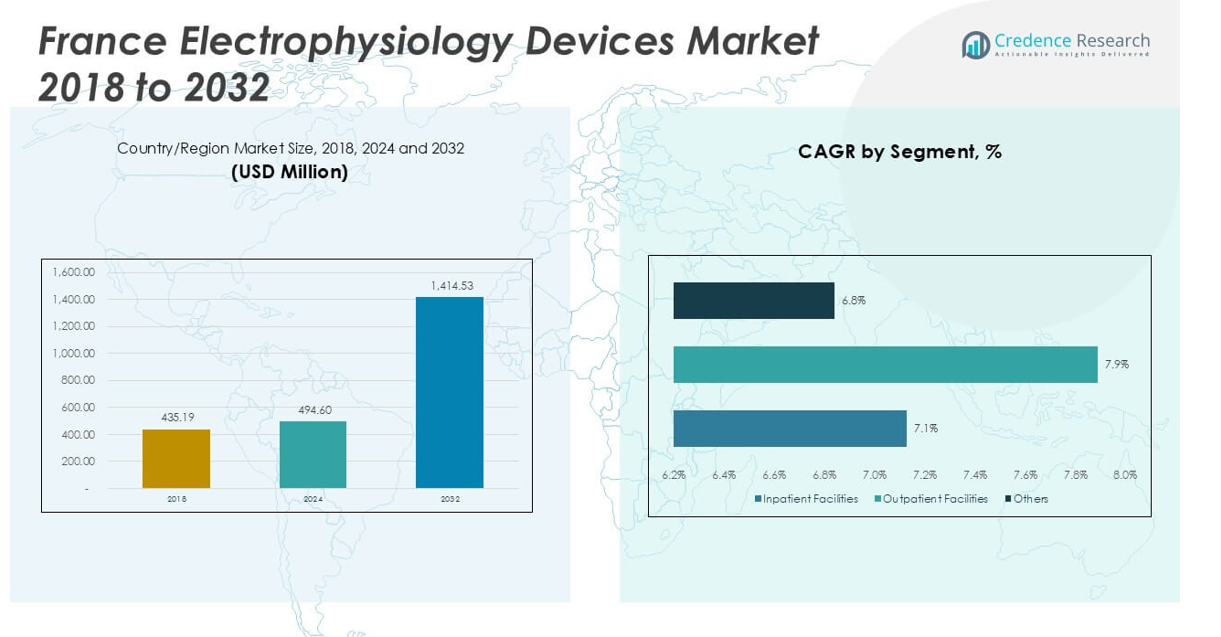
France Electrophysiology Devices Market size was valued at USD 435.19 million in 2023 to USD 494.60 million in 2024 and is anticipated to reach USD 1,414.53 million by 2032, at a CAGR of 14.04% during the forecast period.
The France Electrophysiology Devices Market demonstrates robust growth driven by a rising prevalence of cardiac arrhythmias, an aging population, and increasing awareness of advanced treatment options. Expanding adoption of minimally invasive electrophysiology procedures and continuous technological innovations—such as 3D mapping systems and next-generation ablation catheters—are fueling market expansion. Hospitals and specialized cardiac centers are investing in state-of-the-art equipment to enhance procedural accuracy and patient outcomes. Favorable government initiatives and healthcare reimbursement policies are improving patient access to electrophysiology treatments, further supporting market growth. Leading manufacturers are focusing on strategic collaborations and product launches to strengthen their market presence. Notably, the market benefits from growing physician expertise in complex arrhythmia management and an increasing preference for early diagnosis and intervention. Collectively, these trends and drivers are shaping a highly competitive, innovation-led landscape for electrophysiology devices in France, positioning the market for continued expansion through the forecast period.
Geographical analysis of the France Electrophysiology Devices Market reveals significant activity concentrated in urban regions such as Northern and Southern France, where advanced healthcare infrastructure and specialized cardiac centers drive procedural volumes and technology adoption. Major cities like Paris, Marseille, and Toulouse serve as leading hubs for clinical research, innovation, and patient access, while regions like Eastern and Western France continue to expand their capabilities through investments in modern electrophysiology labs and telemedicine platforms. The market’s competitive landscape is shaped by prominent players including Biosense Webster Inc., Medtronic PLC, and Abbott Laboratories. These companies set industry standards through continuous product innovation, strong research and development pipelines, and strategic partnerships with local hospitals and academic institutions. Their efforts ensure that French healthcare providers have access to the latest electrophysiology technologies, supporting improved patient outcomes and further market growth.
Market Insights
- France Electrophysiology Devices Market reached USD 494.60 million in 2024 and is projected to grow to USD 1,414.53 million by 2032, at a CAGR of 14.04%.
- Rising incidence of cardiac arrhythmias and an aging population are primary drivers boosting demand for advanced electrophysiology devices across the country.
- The market is experiencing a clear trend toward adoption of minimally invasive ablation procedures, integration of 3D mapping systems, and increased use of artificial intelligence in clinical workflows.
- Key players such as Biosense Webster Inc., Medtronic PLC, and Abbott Laboratories maintain a strong competitive presence through robust product portfolios, research investments, and local partnerships.
- High device costs and limited access in rural regions act as restraints, creating challenges for equitable market expansion and timely patient care outside urban centers.
- Northern France leads in device adoption due to superior healthcare infrastructure, while Southern and Eastern France continue to advance with investments in cardiac care networks and technology upgrades; Western France remains an emerging region focused on expanding procedural capacity.
- Continuous government support, favorable reimbursement frameworks, and a national emphasis on innovation and training contribute to sustained market growth and adoption of next-generation electrophysiology solutions.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers
Rising Prevalence of Cardiac Arrhythmias and an Aging Population Fuel Demand
France Electrophysiology Devices Market is experiencing heightened demand due to an increasing prevalence of cardiac arrhythmias, particularly among the elderly. A growing elderly demographic has contributed to a higher incidence of atrial fibrillation and other arrhythmias, creating a need for advanced diagnostic and treatment solutions. Healthcare providers are prioritizing early detection and intervention, making electrophysiology devices critical in managing these conditions. Hospitals and clinics are witnessing a steady flow of patients requiring monitoring and ablation procedures. It responds to the evolving clinical landscape by delivering solutions tailored to both routine and complex arrhythmia cases. The expanding patient base continues to drive investments in electrophysiology infrastructure across the country.
- For instance, cardiovascular diseases remain the leading cause of death in France, accounting for nearly 140,000 deaths annually, with atrial fibrillation affecting approximately 1 million people nationwide.
Technological Advancements and Shift Toward Minimally Invasive Procedures Drive Market Growth
Continuous technological progress plays a pivotal role in the development of the France Electrophysiology Devices Market. Next-generation mapping systems, advanced ablation catheters, and integration of artificial intelligence are elevating procedural success rates and safety profiles. Hospitals and cardiac centers are equipping their electrophysiology labs with cutting-edge technology to offer minimally invasive procedures, resulting in shorter recovery times and improved patient comfort. It enables healthcare providers to perform complex interventions with greater precision and efficiency. Demand for minimally invasive solutions aligns with patient preferences and modern clinical practice standards. The market benefits from ongoing research and a steady stream of innovative product launches.
- For instance, the adoption of robotic-assisted electrophysiology procedures has increased by 45%, improving procedural precision and patient outcomes.
Government Support and Favorable Reimbursement Policies Bolster Market Expansion
The role of government initiatives and favorable reimbursement structures cannot be overlooked in the growth of the France Electrophysiology Devices Market. French healthcare authorities support advancements in cardiac care through funding and regulatory incentives. It allows hospitals and clinics to upgrade their equipment and expand access to electrophysiology services. Streamlined reimbursement policies make it financially viable for patients to seek early diagnosis and treatment. These supportive measures ensure that a wider segment of the population benefits from state-of-the-art electrophysiology procedures. Policy-driven improvements in healthcare delivery create a robust foundation for future growth.
Strategic Industry Collaborations and Focus on Physician Training Enhance Market Competitiveness
Collaboration among medical device manufacturers, hospitals, and research institutions strengthens the France Electrophysiology Devices Market. Companies form alliances to accelerate product development, conduct clinical trials, and address evolving clinical needs. It encourages ongoing education and training for physicians, ensuring widespread adoption of advanced techniques. The industry’s commitment to supporting clinician expertise drives successful outcomes and patient satisfaction. Strategic partnerships also foster innovation, leading to the introduction of novel electrophysiology devices in the French market. Such initiatives reinforce the competitive landscape and support sustainable market expansion.
Market Trends
Rising Adoption of Advanced Mapping and Navigation Technologies Shapes Market Dynamics
The France Electrophysiology Devices Market is witnessing a surge in the adoption of advanced cardiac mapping and navigation technologies. Hospitals and cardiac centers are integrating sophisticated 3D mapping systems and real-time imaging tools to enhance procedural accuracy and patient safety. It allows physicians to localize arrhythmogenic foci with greater precision, supporting more effective ablation therapies. Demand for these solutions reflects a broader trend toward digitalization and data-driven clinical decision-making in electrophysiology. Device manufacturers are investing in continuous improvements to offer intuitive user interfaces and compatibility with various procedural workflows. The integration of such technologies is reshaping clinical practice standards and setting new benchmarks for patient care.
- For instance, advanced 3D mapping systems generate precise, real-time maps of the electrical activity of the heart, improving catheter placement accuracy during ablation treatments.
Growth in Minimally Invasive and Outpatient Electrophysiology Procedures Gains Traction
Shifting preferences among patients and healthcare providers have led to a rise in minimally invasive and outpatient electrophysiology procedures. The France Electrophysiology Devices Market supports this trend by offering devices tailored to shorter hospital stays and faster recovery periods. It facilitates procedures such as catheter ablation and diagnostic electrophysiology studies with minimal disruption to patients’ daily lives. Hospitals benefit from reduced resource utilization and improved operational efficiency. The growing outpatient segment aligns with healthcare system priorities to optimize costs and enhance patient experiences. Market participants are responding by launching compact, user-friendly devices that meet the needs of ambulatory care settings.
- For instance, minimally invasive catheter ablation procedures have increased significantly due to their effectiveness in restoring normal heart rhythm.
Personalized Medicine and Integration of Artificial Intelligence Transform Clinical Outcomes
Personalized medicine and the integration of artificial intelligence are redefining the landscape of the France Electrophysiology Devices Market. Medical centers are leveraging AI-powered algorithms to analyze complex cardiac data and predict patient responses to various treatments. It empowers clinicians to tailor therapies based on individual risk profiles and optimize procedural strategies. Customization extends to device selection, with manufacturers offering modular and adaptable platforms. AI-driven decision support tools are becoming standard in many electrophysiology labs, accelerating clinical workflow and improving diagnostic accuracy. These trends position the market at the forefront of precision healthcare in cardiac electrophysiology.
Sustainable and Eco-Friendly Device Solutions Gain Momentum
Growing awareness of environmental sustainability has prompted manufacturers in the France Electrophysiology Devices Market to pursue eco-friendly product designs. Companies are exploring biodegradable materials, energy-efficient production methods, and recyclable packaging to reduce the environmental impact of their devices. It aligns with broader healthcare industry efforts to minimize waste and adopt responsible supply chain practices. Healthcare providers are increasingly seeking suppliers who demonstrate a commitment to sustainability. This focus on green innovation is influencing purchasing decisions and shaping long-term market strategies. Sustainability remains a key differentiator in a competitive marketplace, driving ongoing development in eco-conscious device solutions.
Market Challenges Analysis
High Cost of Devices and Limited Access in Rural Regions Restrain Market Growth
The France Electrophysiology Devices Market faces challenges from the high cost of advanced equipment and limited accessibility in rural areas. Many healthcare facilities encounter budgetary constraints that slow the adoption of cutting-edge technologies. It limits the reach of state-of-the-art devices to larger urban hospitals while smaller or rural centers struggle to provide comprehensive electrophysiology care. Patients in remote regions often travel long distances for specialized procedures, which creates delays in timely diagnosis and treatment. Disparities in healthcare infrastructure across the country reinforce these accessibility gaps. The market must address cost barriers to ensure equitable access and broader market penetration.
- For instance, the initial capital investment required for setting up electrophysiology laboratories and the cost of procedures can be a significant barrier, limiting accessibility for some healthcare providers and patients.
Regulatory Hurdles and Complex Reimbursement Processes Impede Innovation
Stringent regulatory requirements and complex reimbursement frameworks present persistent challenges for the France Electrophysiology Devices Market. Medical device manufacturers navigate rigorous approval processes that extend time to market for new technologies. It increases development costs and complicates the introduction of innovative products. Hospitals and clinics also contend with evolving reimbursement criteria, which may not always align with the latest clinical advancements. Delays in regulatory approval and reimbursement decisions affect both manufacturers and healthcare providers. These challenges require coordinated efforts among industry stakeholders, regulators, and payers to foster a supportive environment for innovation and growth.
Market Opportunities
Expansion of Telemedicine and Remote Monitoring Solutions Opens New Growth Avenues
The France Electrophysiology Devices Market presents significant opportunities through the expansion of telemedicine and remote monitoring solutions. Hospitals and clinics are exploring digital health platforms that support the continuous monitoring of patients with arrhythmias, enabling earlier intervention and improved outcomes. It leverages wearable and implantable devices that transmit real-time cardiac data to healthcare professionals, reducing the need for frequent hospital visits. Telehealth adoption aligns with government initiatives to modernize healthcare infrastructure and improve access, especially for patients in remote regions. Device manufacturers can partner with digital health companies to offer integrated solutions. This convergence of electrophysiology and digital health is set to reshape patient management and drive market growth.
Rising Investments in Research, Training, and Cross-Industry Collaboration Accelerate Innovation
Rising investments in research, clinical training, and cross-industry partnerships create new opportunities for the France Electrophysiology Devices Market. Leading manufacturers and research institutions are collaborating to develop next-generation devices with enhanced safety and efficacy. It benefits from increased funding for educational programs that train healthcare professionals in advanced electrophysiology procedures and technologies. Focus on upskilling clinicians supports wider adoption of complex techniques and improves patient outcomes. Cross-industry collaborations with biotechnology and artificial intelligence firms accelerate product innovation and customization. This spirit of partnership and commitment to continuous improvement positions the market for dynamic growth and technological leadership.
Market Segmentation Analysis:
By Type:
The France Electrophysiology Devices Market features a diverse product portfolio, with ablation catheters representing a leading segment. Within ablation catheters, radiofrequency (RF) ablation holds a dominant share due to its established safety and efficacy for treating various arrhythmias. Cryoablation is gaining traction for its precision and lower risk of collateral tissue damage, particularly in atrial fibrillation procedures. Pulse field ablation is emerging as an innovative solution, attracting attention for its ability to selectively ablate cardiac tissue while minimizing complications. Diagnostic catheters play a vital role in mapping and identifying arrhythmogenic sites, supporting accurate and personalized therapies. Laboratory devices, including mapping systems and recording instruments, are essential for procedural success and data-driven clinical decisions. Access devices, used for vascular entry and navigation, round out the segment by enabling smooth and safe procedural workflows.
By Indication:
Device utilization in the France Electrophysiology Devices Market is largely driven by the management of atrial fibrillation (AF). AF remains the most frequently treated arrhythmia, prompting widespread use of ablation and diagnostic devices. Hospitals and specialized cardiac centers focus on offering comprehensive solutions for both initial AF management and complex repeat procedures. Non-atrial fibrillation indications, such as ventricular tachycardia and supraventricular tachycardia, also require advanced electrophysiology devices, though they represent a smaller market segment. It ensures that diverse clinical needs are met with a broad array of devices tailored to specific arrhythmias.
By End-Use: Inpatient Facilities Dominate, Outpatient Segment Expands
Inpatient facilities account for the largest share in the France Electrophysiology Devices Market, reflecting the high volume of complex procedures and the need for advanced infrastructure. Hospitals and large cardiac centers invest heavily in state-of-the-art devices and skilled clinical teams to manage both routine and advanced electrophysiology interventions. Outpatient facilities represent a growing segment, driven by advancements in minimally invasive techniques that allow safe and efficient procedures outside of traditional hospital settings. The market sees an emerging trend toward ambulatory and day-care centers offering specialized electrophysiology services. Other end users, such as research institutes and training centers, also contribute to device demand by focusing on innovation, clinical trials, and education.
Segments:
Based on Type:
- Ablation Catheters
- Radiofrequency (RF) Ablation
- Cryoablation
- Pulse Field Ablation
- Diagnostic Catheters
- Laboratory Devices
- Access Devices
Based on Indication:
- Atrial Fibrillation (AF)
- Non-Atrial Fibrillation
Based on End-Use:
- Inpatient Facilities
- Outpatient Facilities
- Others
Based on the Geography:
- Northern France
- Southern France
- Eastern France
- Western France
Regional Analysis
Northern France
Northern France leads the France Electrophysiology Devices Market, accounting for approximately 36% of the total market share. The region’s strong performance stems from a concentration of advanced healthcare infrastructure, large teaching hospitals, and renowned cardiac centers. Major urban hubs such as Paris drive high procedural volumes, supported by extensive investment in cutting-edge electrophysiology laboratories and specialist teams. Northern France has emerged as a leader in clinical research and adoption of next-generation technologies, including 3D mapping systems and AI-assisted ablation devices. Strong collaborations between healthcare providers, research institutes, and device manufacturers support ongoing innovation and training, further reinforcing the region’s leadership position. The presence of national centers of excellence and robust funding for cardiac care ensure that Northern France maintains its dominant role in device utilization and patient outcomes.
Southern France
Southern France holds around 29% of the France Electrophysiology Devices Market, positioning it as the second-largest regional market. The area’s strength lies in its commitment to innovation and the development of specialized cardiac care networks across cities like Marseille, Nice, and Toulouse. Hospitals in Southern France prioritize investment in minimally invasive electrophysiology procedures, attracting both domestic and international patients. The region benefits from a favorable climate for medical tourism, which boosts procedural volumes and demand for advanced devices. Healthcare authorities in Southern France actively support the expansion of outpatient cardiac centers, making high-quality arrhythmia care more accessible to a broader population. The local emphasis on patient-centric approaches and integration of telehealth solutions drives further growth in market share. Southern France continues to strengthen its position through targeted investments in physician training and adoption of new device technologies.
Eastern France
Eastern France captures about 20% of the France Electrophysiology Devices Market, reflecting its steady growth trajectory. The region is characterized by expanding healthcare networks and a rising number of specialized centers offering advanced electrophysiology services. Cities like Strasbourg and Nancy are at the forefront of incorporating modern laboratory devices and ablation technologies into routine clinical practice. Eastern France benefits from cross-border collaboration with neighboring countries, facilitating knowledge exchange and adoption of best practices in cardiac care. Regional governments and healthcare systems are investing in infrastructure upgrades to address the needs of both urban and semi-rural populations. Eastern France’s balanced focus on research, clinical excellence, and accessibility supports a positive outlook for sustained market expansion and improved patient outcomes.
Western France
Western France represents approximately 15% of the France Electrophysiology Devices Market, occupying a niche but steadily expanding position. The region is home to several emerging centers specializing in cardiac arrhythmia management, particularly in cities such as Nantes and Bordeaux. Western France’s healthcare providers have prioritized the adoption of laboratory devices, diagnostic catheters, and minimally invasive ablation solutions. Ongoing investment in hospital infrastructure and physician education is improving procedural capacity and elevating the region’s clinical standards. Western France has also begun to leverage telemedicine platforms to bridge gaps in access for remote and rural populations, driving incremental growth in market penetration. The region’s strategy to build robust cardiac care capabilities, combined with targeted public health initiatives, supports a favorable environment for the continued adoption of electrophysiology devices.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Biosense Webster Inc.
- Medtronic PLC
- Abbott Laboratories
- Boston Scientific Corp
- Biotronik AG
- Siemens Healthcare AG
Competitive Analysis
The competitive landscape of the France Electrophysiology Devices Market is defined by a few leading players: Biosense Webster Inc., Medtronic PLC, Abbott Laboratories, Boston Scientific Corp, Biotronik AG, and Siemens Healthcare AG. These companies maintain a dominant presence through their broad product portfolios, commitment to research and development, and close collaborations with French healthcare institutions. Leading companies in this sector focus on expanding their product portfolios, integrating advanced mapping systems, and developing minimally invasive solutions to meet evolving clinical needs. They invest heavily in research and development, driving the introduction of next-generation devices and maintaining high standards for safety and efficacy. The market benefits from collaborations with local hospitals, research centers, and academic institutions, which accelerate clinical trials and support education for healthcare professionals. Competitive strategies also include strengthening distribution networks and enhancing after-sales service to ensure timely support and reliable device performance. Market participants respond proactively to regulatory changes and reimbursement dynamics, maintaining flexibility and adaptability in their business models. This intense competition fosters continuous improvement, ensuring that healthcare providers in France have access to state-of-the-art electrophysiology technologies and best-in-class patient care.
Recent Developments
- In August 2023, Biosense Webster received approval for various atrial fibrillation ablation products that can be utilized in a workflow without fluoroscopy during catheter ablation procedures.
- In August 2023, Boston Scientific Corporation (US) launched the POLARx cryoablation system. This system is used to treat patients with paroxysmal atrial fibrillation.
- In May 2023 Abbott Laboratories launched the Tactiflex ablation catheter which is sensor-enabled and it is used to treat the most common abnormal heart rhythm.
Market Concentration & Characteristics
The France Electrophysiology Devices Market displays a moderate to high level of market concentration, with a few multinational companies holding significant shares due to their technological leadership and comprehensive product offerings. It exhibits characteristics of rapid innovation, driven by constant advancements in mapping systems, ablation techniques, and integration of digital health solutions. The market places strong emphasis on clinical efficacy, patient safety, and regulatory compliance, ensuring that only high-quality devices are widely adopted. Hospitals and cardiac centers across France prioritize investment in state-of-the-art equipment and value-added services, reflecting a demand for precision and efficiency in electrophysiology procedures. The market also benefits from collaborative relationships between manufacturers, healthcare providers, and research institutions, which accelerate adoption of new technologies and support ongoing medical education. This environment fosters continuous improvement and sets high benchmarks for performance and patient outcomes within the sector.
Report Coverage
The research report offers an in-depth analysis based on Type, Indication, End-Use and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The France Electrophysiology Devices Market is projected to experience significant growth, driven by the increasing prevalence of cardiac arrhythmias and advancements in medical technology.
- Ablation catheters are expected to remain the leading product segment, with innovations enhancing procedural efficacy and patient safety.
- The adoption of minimally invasive procedures is anticipated to rise, supported by technological advancements and patient preference for reduced recovery times.
- Integration of artificial intelligence and machine learning in electrophysiology is likely to improve diagnostic accuracy and personalize treatment plans.
- Telemedicine and remote monitoring solutions are expected to expand, facilitating continuous patient care and early detection of arrhythmias.
- Government initiatives and favorable reimbursement policies are projected to support market expansion and improve access to advanced electrophysiology treatments.
- Collaborations between medical device manufacturers and healthcare providers are anticipated to drive innovation and streamline the adoption of new technologies.
- The aging population in France is likely to contribute to increased demand for electrophysiology procedures and devices.
- Educational programs and training for healthcare professionals are expected to enhance the proficiency in utilizing advanced electrophysiology equipment.
- The market is poised for growth through continuous research and development efforts, aiming to introduce novel solutions for effective arrhythmia management.





