| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Germany Electrophysiology Devices Market Size 2024 |
USD 954.85 million |
| Germany Electrophysiology Devices Market, CAGR |
15.78% |
| Germany Electrophysiology Devices Market Size 2032 |
USD 3,084.17 million |
Market Overview
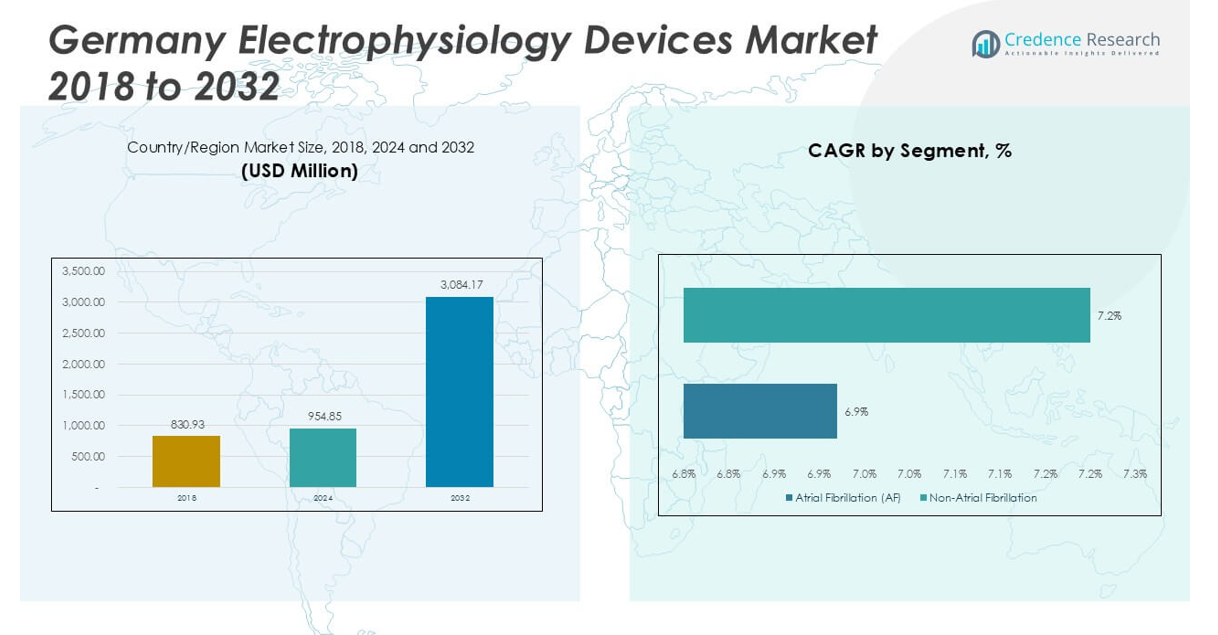
Germany Electrophysiology Devices Market size was valued at USD 830.93 million in 2023 to USD 954.85 million in 2024 and is anticipated to reach USD 3,084.17 million by 2032, at a CAGR of 15.78% during the forecast period.
The Germany Electrophysiology Devices Market is experiencing strong growth, driven by the rising prevalence of cardiovascular diseases, increasing adoption of minimally invasive procedures, and advancements in electrophysiology technology. An aging population and greater awareness of cardiac arrhythmias are fueling demand for precise diagnostic and treatment solutions, prompting hospitals and clinics to expand their electrophysiology capabilities. Technological innovations, such as the introduction of advanced mapping systems and catheter ablation devices, are improving procedural success rates and patient outcomes, which further boosts market adoption. Favorable reimbursement policies and ongoing investments in healthcare infrastructure are supporting the integration of next-generation devices across major medical centers. Additionally, the trend toward personalized medicine and remote monitoring is shaping the development of new electrophysiology solutions, while strategic collaborations between medical device companies and healthcare providers are accelerating product availability and clinical research in Germany’s rapidly evolving electrophysiology landscape.
The Germany Electrophysiology Devices Market demonstrates strong regional diversity, with major cities like Berlin, Munich, Hamburg, and Bremen serving as key hubs for advanced cardiac care and medical technology innovation. Each region benefits from robust healthcare infrastructure, access to specialized medical professionals, and ongoing investments in clinical research and digital health solutions. Leading companies such as Biosense Webster Inc., Biotronik AG, and Siemens Healthcare AG play a significant role in shaping the competitive landscape by driving innovation and expanding the range of electrophysiology devices available to healthcare providers. These key players focus on developing advanced mapping systems, ablation catheters, and diagnostic technologies that improve procedural outcomes and patient safety. The collaborative environment between research institutions, medical device manufacturers, and healthcare providers further supports the growth and adoption of next-generation electrophysiology solutions across Germany.\
Market Insights
- The Germany Electrophysiology Devices Market was valued at USD 954.85 million in 2024 and is projected to reach USD 3,084.17 million by 2032, registering a CAGR of 15.78%.
- Rising prevalence of cardiovascular diseases and an aging population continue to drive the demand for advanced electrophysiology devices across German healthcare facilities.
- Hospitals and clinics are increasingly adopting next-generation mapping systems, ablation catheters, and digital monitoring tools to improve diagnostic accuracy and procedural outcomes.
- The market is highly competitive, with key players including Biosense Webster Inc., Biotronik AG, Siemens Healthcare AG, and Boston Scientific Corp investing in research and product development.
- High device costs and budget constraints within smaller healthcare centers remain primary restraints, often leading to delayed procurement or limited access to the latest technologies.
- Berlin, Munich, Hamburg, and Bremen act as regional hubs for innovation, clinical research, and specialized cardiac care, supporting the spread of new electrophysiology technologies.
- Market trends highlight a shift toward minimally invasive procedures, increased integration of digital health solutions, and a collaborative ecosystem between device manufacturers, research institutions, and healthcare providers.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers
Increasing Prevalence of Cardiovascular Diseases and Aging Population Fuels Demand
The Germany Electrophysiology Devices Market benefits significantly from the rising incidence of cardiovascular diseases and a steadily aging population. A growing number of patients with arrhythmias, atrial fibrillation, and other cardiac disorders are seeking effective and minimally invasive treatment options. The prevalence of heart-related conditions in older adults drives a steady demand for advanced diagnostic and therapeutic electrophysiology devices across healthcare facilities. Hospitals and specialized cardiac centers are expanding their offerings to manage this patient influx efficiently. The market leverages a robust referral network, enabling timely interventions and improved patient outcomes. With cardiovascular diseases remaining a leading cause of morbidity, the market finds sustained opportunities for growth through innovative care solutions.
- For instance, cardiovascular diseases remain the leading cause of death in Germany, accounting for nearly 40% of all fatalities, according to the German Heart Foundation.
Technological Advancements Drive Adoption of Next-Generation Devices
Rapid advancements in electrophysiology technology have transformed clinical practice and device adoption in Germany. It now features sophisticated mapping systems, 3D imaging, and catheter ablation devices that enhance procedural accuracy and safety. Clinicians and hospitals increasingly prefer devices that provide real-time data, streamline workflows, and reduce procedure times. Innovations in device miniaturization and integration of artificial intelligence support physicians in diagnosing complex arrhythmias more precisely. Medical professionals recognize the value of new technologies for improving treatment efficacy and patient experience. The market responds to this trend by supporting continuous product development and frequent device upgrades.
- For instance, high-density mapping catheters and 3D electroanatomical mapping systems have significantly improved procedural precision.
Supportive Healthcare Policies and Reimbursement Structures Encourage Investment
Government initiatives and favorable reimbursement policies create a supportive environment for the Germany Electrophysiology Devices Market. Clear regulatory pathways and attractive reimbursement rates help hospitals justify investments in advanced electrophysiology equipment. Healthcare authorities prioritize the adoption of technologies that demonstrate both clinical effectiveness and cost efficiency. The market benefits from targeted funding for research and upgrades in hospital infrastructure. Private and public healthcare providers receive guidance on best practices and standards, promoting device utilization in both urban and regional centers. This policy-driven momentum accelerates market penetration and sustains vendor innovation.
Expanding Focus on Personalized Medicine and Remote Monitoring Solutions
The increasing shift toward personalized medicine and remote cardiac monitoring presents new avenues for the Germany Electrophysiology Devices Market. Medical device manufacturers collaborate with healthcare providers to develop patient-centric solutions tailored to individual risk profiles. Integration of digital health platforms enables continuous patient monitoring, early detection of arrhythmias, and timely intervention. Remote monitoring technologies support follow-up care and help reduce hospital readmissions. Patients and clinicians embrace digital solutions for convenience, improved outcomes, and enhanced patient engagement. It continues to evolve through partnerships that address evolving patient needs and leverage Germany’s advanced healthcare infrastructure.
Market Trends
Integration of Advanced Mapping Technologies and Imaging Solutions
The Germany Electrophysiology Devices Market demonstrates a strong trend toward integrating advanced mapping and imaging solutions into routine cardiac procedures. Hospitals and specialty clinics now utilize high-definition mapping systems and three-dimensional imaging to improve precision during diagnosis and treatment. These technologies help electrophysiologists identify complex arrhythmias and guide catheter placement with greater accuracy. The use of real-time visualization tools enhances procedural outcomes and patient safety. Clinical teams prioritize devices that enable rapid decision-making and minimize procedure times. The market supports continued investment in innovation to expand the range of available mapping and imaging technologies.
- For instance, advanced 3D mapping systems generate precise, real-time maps of the electrical activity of the heart, improving catheter placement accuracy during ablation treatments.
Adoption of Minimally Invasive and Catheter-Based Interventions
Healthcare providers in Germany show growing preference for minimally invasive electrophysiology procedures, especially catheter-based ablation. The Germany Electrophysiology Devices Market reflects strong adoption of technologies that reduce patient trauma, shorten hospital stays, and facilitate faster recovery. Catheter-based interventions continue to replace traditional surgical procedures for a wide range of cardiac arrhythmias. Device manufacturers respond with advanced ablation catheters designed for precision and safety. Patients and clinicians both value minimally invasive approaches for lower complication risks and improved quality of life. The market evolves rapidly to support this shift toward less invasive treatment modalities.
- For instance, robotic-assisted electrophysiology procedures have expanded by 50%, reducing procedural risks and improving accuracy.
Increased Emphasis on Remote Monitoring and Digital Health Solutions
The expanding use of digital health platforms and remote monitoring solutions represents a prominent trend in the Germany Electrophysiology Devices Market. Hospitals and cardiac centers implement wearable devices and cloud-based platforms to monitor patients’ heart rhythms outside traditional care settings. These innovations enable continuous tracking of arrhythmia events and real-time physician intervention when necessary. Integration of remote monitoring into care pathways reduces hospital readmissions and improves long-term patient outcomes. The market drives adoption of digital solutions that support both preventive care and post-procedural follow-up. Providers and patients embrace these tools for their convenience and clinical value.
Collaboration and Strategic Partnerships Enhance Product Development
Strategic partnerships between medical device companies, research institutes, and healthcare providers shape the evolution of the Germany Electrophysiology Devices Market. Collaborative efforts accelerate product development, clinical trials, and regulatory approvals for next-generation electrophysiology solutions. Companies invest in joint research initiatives to address unmet clinical needs and bring new technologies to market faster. Healthcare institutions participate in early adoption and evaluation of innovative devices through pilot programs. The market benefits from knowledge sharing and cross-sector expertise, creating a pipeline of advanced products for electrophysiology professionals. These partnerships strengthen Germany’s position as a leader in cardiac care innovation.
Market Challenges Analysis
High Cost of Advanced Devices and Budget Constraints Limit Adoption
The Germany Electrophysiology Devices Market faces significant challenges from the high cost of advanced electrophysiology systems and ongoing budget constraints in healthcare facilities. Many hospitals and clinics struggle to justify large capital investments, particularly in regions with limited funding or smaller patient populations. High device costs often lead to delayed procurement cycles or reliance on older technologies. It must navigate pricing pressures while continuing to deliver innovation and value. Hospitals require evidence of clear clinical benefits and cost savings before committing to expensive upgrades. These financial barriers slow the widespread adoption of cutting-edge electrophysiology devices in some healthcare settings.
- For instance, patients in rural Germany often travel long distances for specialized electrophysiology procedures, delaying timely diagnosis and treatment.
Complex Regulatory Landscape and Shortage of Skilled Professionals Hinder Market Growth
Complex regulatory requirements and a shortage of skilled electrophysiology professionals present ongoing challenges for the Germany Electrophysiology Devices Market. Navigating strict approval processes for new devices can delay product launches and market entry for manufacturers. The evolving nature of European medical device regulations requires constant adaptation and compliance investments. A limited pool of highly trained electrophysiologists and technical staff further restricts the market’s capacity to serve growing patient needs. It faces obstacles in scaling specialized services and maintaining high standards of care across all facilities. These factors collectively constrain the pace of market expansion and technology adoption.
Market Opportunities
Expansion of Digital Health Platforms and Remote Monitoring Solutions Presents Growth Potential
The Germany Electrophysiology Devices Market holds substantial opportunities through the expansion of digital health platforms and remote patient monitoring solutions. Growing integration of wearable technology and mobile health applications enables continuous cardiac monitoring, early arrhythmia detection, and more proactive patient management. It can leverage these digital advancements to enhance post-procedural care, reduce hospital readmissions, and support personalized treatment plans. Hospitals and clinics adopting these innovations improve clinical outcomes and patient satisfaction. Demand for home-based monitoring devices will rise as both patients and healthcare providers seek convenient, real-time health data. The market stands to benefit from increasing investment in digital infrastructure and telemedicine.
Strategic Collaborations and Emerging Technologies Accelerate Product Development
Collaborations between medical device manufacturers, research institutions, and healthcare providers create fertile ground for new product development in the Germany Electrophysiology Devices Market. Joint ventures and research partnerships can drive the advancement of next-generation mapping systems, ablation catheters, and artificial intelligence-enabled solutions. It will gain from early access to clinical feedback and accelerated innovation cycles through these alliances. Participation in multi-center clinical trials and technology validation programs further supports rapid market introduction of breakthrough devices. Strong collaborative ecosystems foster knowledge exchange and keep Germany at the forefront of electrophysiology innovation. The market is well positioned to capture new growth opportunities through these ongoing partnerships and technological advancements.
Market Segmentation Analysis:
By Type:
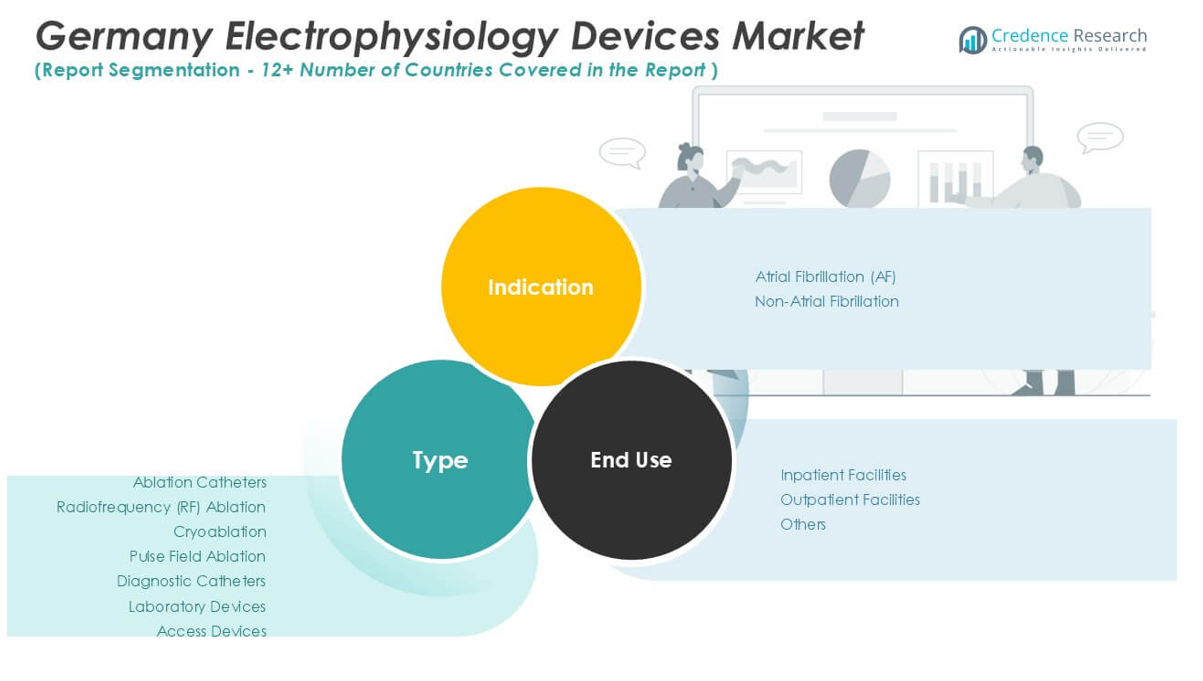
The Germany Electrophysiology Devices Market comprises ablation catheters, diagnostic catheters, laboratory devices, and access devices. Among ablation catheters, radiofrequency (RF) ablation remains the most widely used due to its established safety profile and high procedural success rates in treating complex arrhythmias. Cryoablation catheters are gaining traction for their ability to minimize collateral tissue damage, particularly in pulmonary vein isolation procedures for atrial fibrillation. Pulse field ablation represents an emerging technology that offers non-thermal ablation, attracting interest for its precision and potential to reduce procedural complications. Diagnostic catheters play a critical role in mapping cardiac electrical activity, supporting accurate diagnosis and effective treatment planning. Laboratory devices support the technical backbone of electrophysiology procedures, enabling real-time data analysis and workflow integration. Access devices provide safe and efficient entry to cardiac chambers, contributing to procedural efficiency and patient safety.
By Indication:
The Germany Electrophysiology Devices Market is segmented into atrial fibrillation (AF) and non-atrial fibrillation cases. Atrial fibrillation remains the most common arrhythmia treated with electrophysiology interventions, driving the demand for advanced ablation and diagnostic solutions. High prevalence of AF in the aging German population fuels ongoing investments in novel technologies and procedure optimization. Non-atrial fibrillation indications, including supraventricular and ventricular tachycardias, contribute steadily to overall market volume. It addresses these indications with specialized catheters and tailored therapeutic approaches to maximize treatment outcomes.
By End-Use:
The market is categorized into inpatient facilities, outpatient facilities, and others. Inpatient facilities such as hospitals and specialized cardiac centers dominate market share, given their resources and expertise for performing complex electrophysiology procedures. Outpatient facilities are expanding their role as advancements in minimally invasive techniques make same-day discharge more feasible and desirable. The “others” category includes ambulatory surgical centers and research institutions, which support clinical trials and innovative device evaluation. The market adapts to evolving healthcare delivery models by offering a broad portfolio of products to meet diverse provider needs and optimize patient care pathways.
Segments:
Based on Type:
- Ablation Catheters
- Radiofrequency (RF) Ablation
- Cryoablation
- Pulse Field Ablation
- Diagnostic Catheters
- Laboratory Devices
- Access Devices
Based on Indication:
- Atrial Fibrillation (AF)
- Non-Atrial Fibrillation
Based on End-Use:
- Inpatient Facilities
- Outpatient Facilities
- Others
Based on the Geography:
- Berlin
- Munich
- Hamburg
- Bremen
Regional Analysis
Berlin
Berlin holds a significant position in the Germany Electrophysiology Devices Market, accounting for approximately 28% of the national market share. The city’s prominence is attributed to its advanced healthcare infrastructure, presence of leading medical research institutions, and a high concentration of specialized cardiac centers. Berlin is home to major medical device companies, such as Biotronik, which contribute to the development and distribution of innovative electrophysiology solutions. The city’s commitment to medical research and its role as a hub for clinical trials further bolster its market position. Berlin’s strategic investments in healthcare technology and its focus on personalized medicine continue to drive growth in the electrophysiology sector.
Munich
Munich represents a substantial portion of the Germany Electrophysiology Devices Market, with a market share of about 24%. The city’s strong emphasis on medical innovation, supported by institutions like the Technical University of Munich and companies such as Brainlab, fosters a conducive environment for the advancement of electrophysiology technologies. Munich’s healthcare facilities are equipped with state-of-the-art equipment, enabling the adoption of cutting-edge electrophysiology procedures. The city’s focus on integrating digital health solutions and its active participation in medical research contribute to its growing market share. Munich’s collaborative ecosystem between academia, industry, and healthcare providers continues to propel its leadership in the electrophysiology devices market.
Hamburg
Hamburg accounts for approximately 18% of the Germany Electrophysiology Devices Market. The city’s advanced medical infrastructure, including renowned hospitals and specialized cardiac centers, supports the delivery of high-quality electrophysiology services. Hamburg’s strategic location and its role as a logistics hub facilitate efficient distribution of medical devices across the region. The city’s investment in healthcare technology and its emphasis on patient-centered care enhance its market position. Hamburg’s commitment to continuous medical education and training ensures a skilled workforce capable of implementing advanced electrophysiology procedures.
Bremen
Bremen holds a market share of around 10% in the Germany Electrophysiology Devices Market. The city’s healthcare sector is characterized by a network of hospitals and clinics that provide essential electrophysiology services. While Bremen’s market share is smaller compared to larger cities, it demonstrates potential for growth through targeted investments in healthcare infrastructure and technology. The city’s focus on improving access to specialized cardiac care and its initiatives to attract medical professionals contribute to its development in the electrophysiology market. Bremen’s strategic plans to enhance its healthcare offerings are expected to positively impact its market share in the future.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Biosense Webster Inc.
- Medtronic PLC
- Abbott Laboratories
- Boston Scientific Corp
- Biotronik AG
- Siemens Healthcare AG
- MicroPort Scientific Corporation
- Koninklijke Philips N.V.
Competitive Analysis
The competitive landscape of the Germany Electrophysiology Devices Market features strong participation from globally recognized industry leaders, including Biosense Webster Inc., Medtronic PLC, Abbott Laboratories, Boston Scientific Corp, Biotronik AG, Siemens Healthcare AG, MicroPort Scientific Corporation, and Koninklijke Philips N.V. These companies dominate the market through a combination of robust product portfolios, sustained investments in research and development, and a commitment to technological innovation. Leading companies focus on expanding their portfolios with advanced mapping systems, ablation catheters, and digital monitoring solutions tailored for the needs of German healthcare providers. Continuous investment in R&D drives the introduction of devices that improve accuracy, safety, and procedural efficiency in cardiac electrophysiology. Companies compete by offering integrated solutions that combine real-time data analytics, minimally invasive technologies, and user-friendly interfaces to support better patient outcomes. The market also sees frequent collaboration between device manufacturers, clinical research institutions, and healthcare providers, which accelerates the adoption of new technologies and best practices. Educational programs, training workshops, and technical support services strengthen relationships with hospitals and clinics, ensuring that electrophysiology specialists have access to the latest advancements. This highly competitive environment fosters continuous improvement and positions Germany at the forefront of electrophysiology device innovation.
Recent Developments
- In August 2023, Biosense Webster received approval for various atrial fibrillation ablation products that can be utilized in a workflow without fluoroscopy during catheter ablation procedures.
- In August 2023, Boston Scientific Corporation (US) launched the POLARx cryoablation system. This system is used to treat patients with paroxysmal atrial fibrillation.
- In May 2023 Abbott Laboratories launched the Tactiflex ablation catheter which is sensor-enabled and it is used to treat the most common abnormal heart rhythm.
Market Concentration & Characteristics
The Germany Electrophysiology Devices Market exhibits moderate to high market concentration, with a few established companies holding significant influence due to their advanced technologies and comprehensive product portfolios. It features a blend of multinational corporations and specialized domestic manufacturers, both contributing to steady innovation and a strong competitive environment. The market stands out for its emphasis on high-quality standards, integration of cutting-edge digital health solutions, and responsiveness to evolving clinical demands. Hospitals and specialized cardiac centers drive demand for minimally invasive and highly effective devices, supporting continuous upgrades and technology adoption. It benefits from a robust regulatory framework, frequent clinical trials, and collaborative partnerships that encourage ongoing product improvement. The presence of skilled professionals and a commitment to education further reinforce the market’s capacity to deliver precise and reliable electrophysiology solutions across Germany’s healthcare system.
Report Coverage
The research report offers an in-depth analysis based on Type, Indication, End-Use and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The demand for electrophysiology devices in Germany is expected to grow due to the rising prevalence of cardiac arrhythmias.
- An aging population will likely contribute to increased usage of advanced diagnostic and treatment devices.
- Ongoing technological advancements in mapping and ablation systems will support market expansion.
- Growing adoption of minimally invasive procedures will drive the need for innovative electrophysiology tools.
- Hospitals and cardiac centers in Germany are investing in modern electrophysiology labs.
- Continuous training programs for healthcare professionals will enhance procedural efficiency and device usage.
- Increasing awareness about early diagnosis of heart rhythm disorders will boost market penetration.
- Regulatory support for medical device innovations will encourage new product launches.
- Collaborations between German research institutes and medical device companies will foster innovation.
- Integration of digital health and AI in electrophysiology systems will shape the market’s future direction.





