| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Temporary Cardiac Pacing Wires Market Size 2024 |
USD 458.30 Million |
| Temporary Cardiac Pacing Wires Market, CAGR |
6.34% |
| Temporary Cardiac Pacing Wires Market Size 2032 |
USD 776.31 Million |
Market Overview
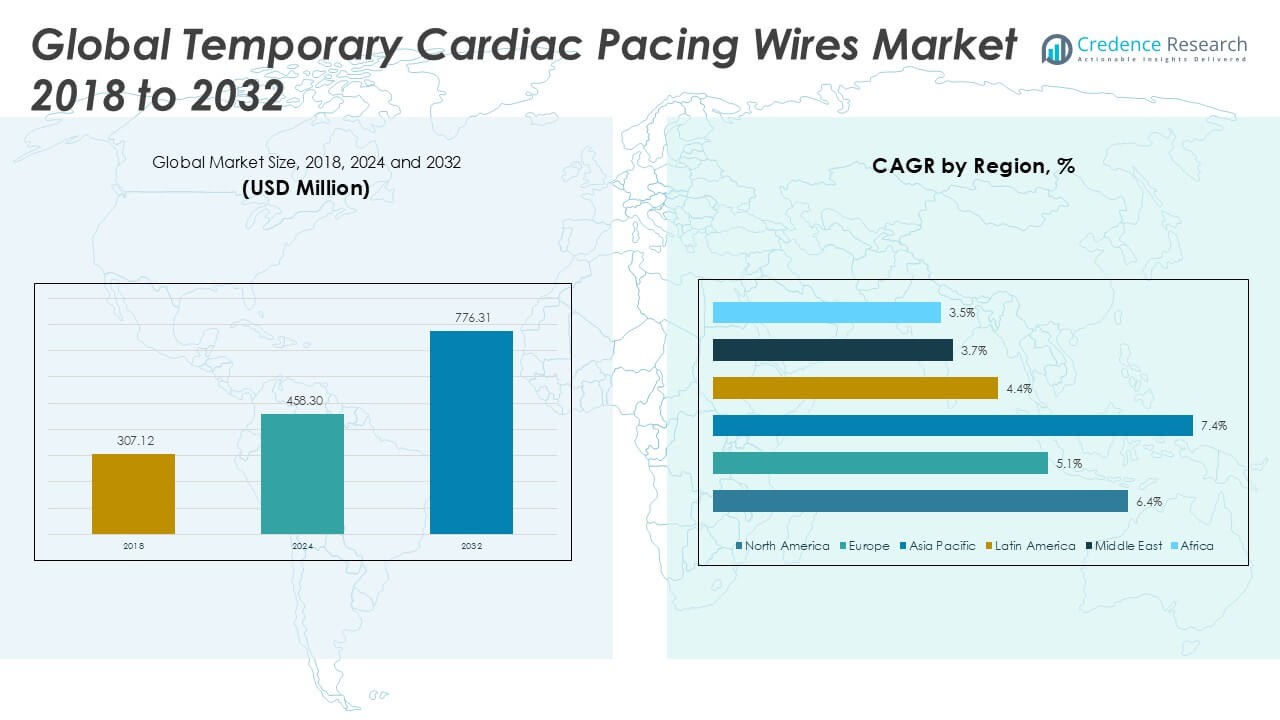
The Temporary Cardiac Pacing Wires Market was valued at USD 307.12 million in 2018 and reached USD 458.30 million in 2024. It is anticipated to reach USD 776.31 million by 2032, reflecting a compound annual growth rate (CAGR) of 6.34% during the forecast period.
The Temporary Cardiac Pacing Wires Market is driven by the rising prevalence of cardiovascular diseases and an increasing number of cardiac surgeries worldwide. The growing geriatric population, which is more susceptible to cardiac conditions, further supports market growth. Advances in pacing technologies and a shift toward minimally invasive cardiac procedures have enhanced the adoption of temporary pacing wires in clinical practice. Hospitals and healthcare providers are increasingly focused on improving post-operative patient outcomes, contributing to demand for reliable pacing solutions. The market also benefits from continuous investments in research and development, resulting in safer and more efficient products. However, the risk of complications and the availability of alternative pacing methods present challenges to market expansion. Emerging economies, with improving healthcare infrastructure and greater access to advanced cardiac care, offer new growth opportunities for manufacturers in the temporary cardiac pacing wires market.
The Temporary Cardiac Pacing Wires Market demonstrates strong presence across major regions including North America, Europe, Asia Pacific, Latin America, the Middle East, and Africa. North America and Europe lead market growth, supported by advanced healthcare infrastructure and high adoption of innovative cardiac care technologies. Asia Pacific shows rapid expansion, driven by rising cardiac disease prevalence and increased investment in healthcare modernization in countries such as China, Japan, and India. Latin America, the Middle East, and Africa present emerging opportunities, with gradual improvements in healthcare access and cardiac care services. Key players shaping the competitive landscape include Medtronic, Abbott Laboratories, and Boston Scientific Corporation. These companies drive product innovation, maintain broad global distribution networks, and support clinical research collaborations that enhance the adoption of temporary cardiac pacing wires in diverse healthcare settings.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The Temporary Cardiac Pacing Wires Market was valued at USD 307.12 million in 2018, reached USD 458.30 million in 2024, and is expected to hit USD 776.31 million by 2032, with a CAGR of 6.34% during the forecast period.
- The market serves as a critical segment within cardiac care, providing temporary pacing support for patients undergoing cardiac surgeries and emergency procedures.
- Rising prevalence of cardiovascular diseases, growing geriatric population, and an increase in the volume of cardiac surgeries are the primary drivers fueling market growth.
- Trends indicate a shift toward the adoption of advanced pacing wire materials, infection-resistant technologies, and greater use of minimally invasive surgical techniques.
- Key players such as Medtronic, Abbott Laboratories, and Boston Scientific Corporation dominate the market, offering innovative product portfolios and strong global distribution capabilities.
- Stringent regulatory requirements, risk of device-related complications, and increasing competition from alternative pacing technologies act as restraints to wider market expansion.
- North America leads the market, followed by Europe and Asia Pacific, while emerging economies in Latin America, the Middle East, and Africa present new growth opportunities driven by expanding healthcare infrastructure and increased focus on cardiac care.
Market Drivers
Rising Prevalence of Cardiovascular Diseases and Surgical Procedures Fuels Demand
The Temporary Cardiac Pacing Wires Market experiences significant momentum due to the increasing incidence of cardiovascular diseases globally. Hospitals perform a higher volume of cardiac surgeries each year, especially with a surge in coronary artery bypass grafting and valve replacement procedures. Temporary pacing wires play a vital role in managing arrhythmias during and after cardiac surgery. Demand grows in both developed and developing regions, reflecting changes in population health and lifestyle factors. Market participants benefit from this expanded patient base, which creates sustained opportunities for growth. Health organizations advocate for early detection and intervention, contributing to a steady requirement for temporary pacing solutions. The market leverages this trend to drive product adoption and expand clinical usage.
- For instance, Medtronic’s temporary cardiac pacing wires have been used in over 10,000 cardiac procedures annually across major hospital networks, supporting reliable intraoperative pacing during high-risk surgeries.
Advances in Cardiac Pacing Technologies Support Market Expansion
Innovations in pacing wire materials and device design enhance the safety and efficacy of temporary cardiac pacing, encouraging broader clinical adoption. The market witnesses a shift toward minimally invasive cardiac interventions, where advanced temporary pacing wires support complex procedures with improved outcomes. Manufacturers invest in developing biocompatible and infection-resistant products, reducing the risk of adverse events for patients. Hospitals and cardiac centers recognize the value of these innovations, accelerating their integration into standard care protocols. Product differentiation helps companies strengthen their market position in a competitive landscape. Continuous improvement in technology ensures the Temporary Cardiac Pacing Wires Market remains dynamic and forward-looking. Healthcare professionals rely on these advancements to ensure optimal patient care.
- For instance, Abbott Laboratories developed a temporary pacing wire featuring a hydrophilic coating, which demonstrated a 38% reduction in post-operative infection rates according to a multicenter clinical trial involving 800 patients.
Growing Geriatric Population Increases Market Potential
A rising elderly population elevates the need for cardiac procedures and temporary pacing solutions. Age-related degeneration of cardiac function leads to more frequent surgical interventions, supporting sustained market demand. The Temporary Cardiac Pacing Wires Market addresses the unique needs of older adults by delivering reliable post-operative pacing support. Geriatric patients often face complex health conditions, requiring advanced cardiac care and temporary pacing during recovery. Hospitals adapt to these demographic shifts by integrating advanced pacing solutions in routine cardiac care. It contributes to improved outcomes for older patients, supporting overall healthcare goals. Market growth aligns closely with demographic trends and advances in geriatric medicine.
Expanding Healthcare Infrastructure in Emerging Markets Drives Adoption
Developing regions invest heavily in modernizing healthcare systems and expanding access to advanced cardiac care. The Temporary Cardiac Pacing Wires Market capitalizes on increased hospital capacity, new surgical centers, and government support for cardiovascular health. Rising healthcare expenditure and awareness about minimally invasive cardiac procedures encourage hospitals to procure advanced pacing wires. Local and multinational manufacturers expand their presence in these regions, offering tailored solutions to meet growing demand. Training programs for clinicians help bridge the skills gap, ensuring safe and effective use of temporary pacing technologies. Market penetration deepens in emerging economies, unlocking new opportunities for growth. It positions manufacturers for long-term success in high-potential regions.
Market Trends
Adoption of Advanced Materials and Infection-Resistant Technologies Shapes Product Development
The Temporary Cardiac Pacing Wires Market features a clear shift toward advanced materials and infection-resistant technologies. Manufacturers introduce wires with improved biocompatibility and reduced risk of device-related infections, responding to clinician and patient safety concerns. Innovations include antimicrobial coatings and specialized polymers that minimize complications. Hospitals recognize the value of these advancements for reducing post-surgical risks, supporting product adoption across major healthcare systems. Product portfolios expand to include single-use and sterile options, aligning with stricter infection control protocols. The trend toward safer and more durable pacing wires continues to influence purchasing decisions. It drives a steady evolution in device quality and clinical performance.
- For instance, Boston Scientific’s single-use, sterile pacing wires have been adopted by over 200 leading cardiac centers, helping reduce device cross-contamination incidents to fewer than 1 per 2,000 uses.
Emphasis on Minimally Invasive Cardiac Procedures Accelerates Wire Utilization
The Temporary Cardiac Pacing Wires Market reflects an increased emphasis on minimally invasive cardiac procedures. Surgeons and interventional cardiologists integrate temporary pacing wires in complex cases where rapid stabilization is critical. Growing patient preference for procedures with shorter recovery times and fewer complications encourages hospitals to adopt new wire technologies. Device makers respond by designing wires that are compatible with catheter-based and less invasive techniques. The trend reinforces the need for versatile pacing solutions that fit a wide range of surgical approaches. Hospitals value the efficiency and reliability of these wires during high-risk interventions. It sustains market growth by supporting evolving clinical practice standards.
- For instance, Biotronik introduced a pacing wire system compatible with transcatheter aortic valve replacement (TAVR) that has been successfully implemented in more than 3,500 minimally invasive heart procedures since launch.
Integration of Digital Monitoring and Remote Management Solutions Enhances Patient Outcomes
The Temporary Cardiac Pacing Wires Market observes a trend toward integrating digital monitoring and remote management capabilities. Medical device firms invest in smart pacing wires that transmit real-time data to clinicians, supporting timely decision-making during recovery. Hospitals benefit from continuous patient monitoring and reduced in-person visits, optimizing post-operative care. The shift to connected devices aligns with the broader movement toward digital health and telemedicine. Data-driven solutions help providers personalize patient management and reduce complications. Remote management features gain traction in large healthcare networks and specialty cardiac centers. It enables efficient care models and enhances patient safety.
Expansion into Emerging Markets Supports Broader Accessibility and Market Penetration
The Temporary Cardiac Pacing Wires Market expands rapidly in emerging markets, where healthcare modernization drives demand for advanced cardiac devices. Manufacturers invest in local partnerships and distribution networks to reach new hospitals and clinics. Awareness programs and training initiatives educate clinicians on the safe use of temporary pacing wires, overcoming barriers to adoption. The market responds to government efforts to improve cardiac care infrastructure and access to minimally invasive treatments. Rising healthcare spending and insurance coverage in these regions support ongoing market expansion. Local adaptation of global technologies ensures relevance and accessibility. It creates a sustainable growth trajectory in untapped markets.
Market Challenges Analysis
Risk of Device-Related Complications and Stringent Regulatory Hurdles Limit Market Growth
The Temporary Cardiac Pacing Wires Market faces challenges from device-related complications such as infection, wire displacement, and cardiac tissue damage. Clinicians express concerns over adverse events during or after pacing, prompting hospitals to evaluate product safety rigorously. Regulatory bodies impose stringent approval standards and post-market surveillance, which can delay new product launches and raise compliance costs. Manufacturers allocate significant resources to clinical testing and documentation to satisfy evolving regulatory demands. Complex and time-consuming certification processes hinder smaller companies’ entry into the market. Market players must address these clinical and regulatory risks to sustain growth and maintain credibility. It navigates a landscape where patient safety and compliance standards continue to intensify.
Competition from Alternative Pacing Technologies and Cost Constraints Impacts Market Adoption
The Temporary Cardiac Pacing Wires Market contends with the growing adoption of alternative pacing technologies such as leadless pacemakers and advanced implantable devices. Healthcare providers increasingly consider long-term solutions for certain patient populations, which reduces reliance on temporary pacing wires. Cost pressures within hospitals and health systems can also limit investment in advanced pacing wires, particularly in price-sensitive markets. Reimbursement policies and budgetary constraints influence purchasing decisions, affecting market penetration for premium products. Manufacturers must justify the value proposition of temporary pacing wires amid competition from technologically advanced alternatives. It faces an ongoing challenge to demonstrate clinical and economic benefits in a dynamic healthcare environment.
Market Opportunities
Risk of Device-Related Complications and Stringent Regulatory Hurdles Limit Market Growth
The Temporary Cardiac Pacing Wires Market faces challenges from device-related complications such as infection, wire displacement, and cardiac tissue damage. Clinicians express concerns over adverse events during or after pacing, prompting hospitals to evaluate product safety rigorously. Regulatory bodies impose stringent approval standards and post-market surveillance, which can delay new product launches and raise compliance costs. Manufacturers allocate significant resources to clinical testing and documentation to satisfy evolving regulatory demands. Complex and time-consuming certification processes hinder smaller companies’ entry into the market. Market players must address these clinical and regulatory risks to sustain growth and maintain credibility. It navigates a landscape where patient safety and compliance standards continue to intensify.
Competition from Alternative Pacing Technologies and Cost Constraints Impacts Market Adoption
The Temporary Cardiac Pacing Wires Market contends with the growing adoption of alternative pacing technologies such as leadless pacemakers and advanced implantable devices. Healthcare providers increasingly consider long-term solutions for certain patient populations, which reduces reliance on temporary pacing wires. Cost pressures within hospitals and health systems can also limit investment in advanced pacing wires, particularly in price-sensitive markets. Reimbursement policies and budgetary constraints influence purchasing decisions, affecting market penetration for premium products. Manufacturers must justify the value proposition of temporary pacing wires amid competition from technologically advanced alternatives. It faces an ongoing challenge to demonstrate clinical and economic benefits in a dynamic healthcare environment.
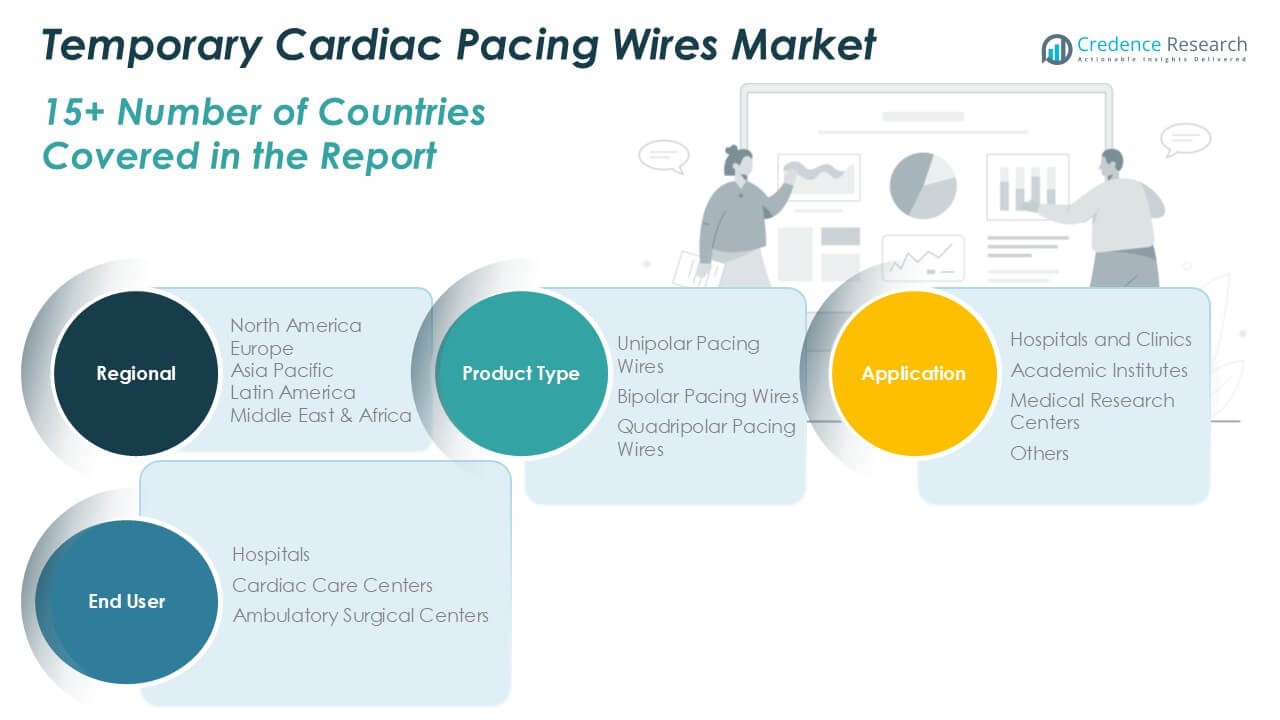
Market Segmentation Analysis:
By Product Type:
Unipolar pacing wires hold a substantial share due to their simplicity and established use in routine cardiac surgeries. Bipolar pacing wires gain traction for their enhanced accuracy and reduced risk of electrical interference, appealing to surgeons seeking reliable intraoperative pacing. Quadripolar pacing wires represent the latest advancement, supporting complex pacing needs with improved flexibility and precision, making them the preferred choice in specialized cardiac procedures.
- For instance, B. Braun Melsungen AG’s quadripolar pacing wires are now routinely used in over 70 advanced cardiac centers worldwide, facilitating multi-chamber pacing during complex arrhythmia surgeries.
By Application:
Hospitals and clinics dominate the market, reflecting the high volume of cardiac surgeries and the need for post-operative pacing in acute care settings. Hospitals rely on a broad portfolio of pacing wires to address diverse patient profiles and clinical scenarios. Academic institutes contribute to the segment by adopting temporary pacing wires for training and procedural research, helping advance cardiac care standards. Medical research centers incorporate these devices into clinical studies, facilitating innovation and evidence-based practice. The ‘others’ category encompasses outpatient facilities and specialty centers that deploy temporary pacing wires for short-term monitoring or emergency stabilization.
- For instance, Teleflex Incorporated reported that its pacing wires have been included in more than 120 clinical research projects globally, leading to the publication of 16 peer-reviewed studies focused on cardiac device safety and efficacy.
By End-User:
Hospitals lead the Temporary Cardiac Pacing Wires Market due to their comprehensive cardiac care infrastructure and high procedural throughput. Cardiac care centers follow, specializing in advanced interventional and surgical cardiology, where temporary pacing wires are integral for managing complex cases. Ambulatory surgical centers present a growing segment, reflecting an industry shift toward minimally invasive procedures and same-day surgeries. It supports faster patient recovery and reduces the length of hospital stays, increasing the adoption of temporary pacing wires in these settings. Each end-user group drives demand with unique clinical priorities, shaping market dynamics and influencing product innovation across the industry.
Segments:
Based on Product Type:
- Unipolar Pacing Wires
- Bipolar Pacing Wires
- Quadripolar Pacing Wires
Based on Application:
- Hospitals and Clinics
- Academic Institutes
- Medical Research Centers
- Others
Based on End User:
- Hospitals
- Cardiac Care Centers
- Ambulatory Surgical Centers
Based on the Geography:
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis
North America Temporary Cardiac Pacing Wires Market
North America Temporary Cardiac Pacing Wires Market grew from USD 122.10 million in 2018 to USD 180.12 million in 2024 and is projected to reach USD 306.04 million by 2032, reflecting a compound annual growth rate (CAGR) of 6.4%. North America is holding a 39.3% market share. The United States and Canada drive regional growth due to advanced healthcare infrastructure, high volume of cardiac procedures, and rapid adoption of innovative pacing technologies. Hospitals and cardiac centers in the U.S. lead the demand, benefiting from strong reimbursement frameworks and a skilled clinical workforce. Clinical research initiatives further support market expansion. The presence of global manufacturers ensures continuous access to next-generation pacing wires in the region.
Europe Temporary Cardiac Pacing Wires Market
Europe Temporary Cardiac Pacing Wires Market grew from USD 60.35 million in 2018 to USD 85.37 million in 2024 and is forecasted to reach USD 132.18 million by 2032, with a CAGR of 5.1%. Europe accounts for a 17% market share. Germany, the UK, France, and Italy represent major contributors, supported by well-established cardiac care services and growing awareness of minimally invasive cardiac treatments. The region emphasizes clinical safety and stringent regulatory standards, influencing product selection and hospital purchasing behavior. Research collaborations between hospitals and academic institutions advance innovation in pacing technology. Market participants capitalize on high procedural volumes and strong demand for advanced cardiac care solutions.
Asia Pacific Temporary Cardiac Pacing Wires Market
Asia Pacific Temporary Cardiac Pacing Wires Market grew from USD 99.92 million in 2018 to USD 156.50 million in 2024 and is anticipated to reach USD 286.55 million by 2032, registering a CAGR of 7.4%. Asia Pacific holds a 37% market share. Key countries include China, Japan, India, South Korea, and Australia, where rapid healthcare modernization and increasing cardiac disease prevalence drive demand. Investments in hospital infrastructure and medical device manufacturing foster market growth. Government health initiatives and insurance coverage improvements expand access to advanced cardiac care. It benefits from a large patient pool and rising adoption of minimally invasive procedures.
Latin America Temporary Cardiac Pacing Wires Market
Latin America Temporary Cardiac Pacing Wires Market grew from USD 12.15 million in 2018 to USD 17.86 million in 2024 and is expected to reach USD 26.10 million by 2032, with a CAGR of 4.4%. Latin America represents a 3% market share. Brazil, Mexico, and Argentina lead market activity, leveraging expanding hospital networks and increased physician training in cardiac care. Investments in healthcare infrastructure support the adoption of modern pacing devices. Regional health policies prioritize early detection and treatment of cardiovascular diseases. Market participants focus on affordability and tailored product offerings to meet local clinical needs.
Middle East Temporary Cardiac Pacing Wires Market
Middle East Temporary Cardiac Pacing Wires Market grew from USD 7.54 million in 2018 to USD 10.15 million in 2024 and is projected to reach USD 14.09 million by 2032, reflecting a CAGR of 3.7%. The Middle East commands a 2% market share. Key countries such as Saudi Arabia, the United Arab Emirates, and Qatar invest in modern hospital infrastructure and cardiovascular specialty centers. Growth remains moderate due to limited procedural volumes compared to developed regions. Government-driven healthcare modernization and medical tourism contribute to market activity. It benefits from partnerships with global manufacturers and increasing demand for advanced cardiac interventions.
Africa Temporary Cardiac Pacing Wires Market
Africa Temporary Cardiac Pacing Wires Market grew from USD 5.06 million in 2018 to USD 8.29 million in 2024 and is forecasted to reach USD 11.35 million by 2032, at a CAGR of 3.5%. Africa holds a 2% market share. South Africa, Egypt, and Nigeria are primary contributors, with demand driven by gradual improvements in healthcare infrastructure and cardiac care awareness. Market growth faces challenges from limited access to specialized cardiac services and affordability issues. International organizations and public health initiatives support capacity building and physician training. It focuses on basic cardiac pacing needs and incremental expansion in key urban centers.
Key Player Analysis
- Medtronic
- Abbott Laboratories
- Boston Scientific Corporation
- Biotronik
- Oscor Inc.
- Braun Melsungen AG
- Teleflex Incorporated
- A&E Medical
- Edwards Lifesciences
- BioTrace Medical
Competitive Analysis
The Temporary Cardiac Pacing Wires Market features a highly competitive landscape led by established medical device manufacturers including Medtronic, Abbott Laboratories, Boston Scientific Corporation, Biotronik, B. Braun Melsungen AG, Teleflex Incorporated, Oscor Inc., A&E Medical, Edwards Lifesciences, and BioTrace Medical. These companies command strong market positions through diverse product portfolios, advanced pacing wire technologies, and global distribution networks. Major companies maintain a strong presence by offering comprehensive portfolios of pacing wires that cater to diverse clinical requirements in cardiac surgery and emergency care. Investment in research and development remains central to their strategies, resulting in advanced features such as biocompatible materials, infection-resistant coatings, and enhanced device flexibility. These innovations support improved patient safety, procedural efficiency, and reduced complication rates. Companies also focus on expanding global distribution networks and forming strategic collaborations with hospitals and research organizations, ensuring broad access to their solutions. Competitive intensity is further heightened by the entrance of specialized firms targeting niche applications and the growing demand for minimally invasive cardiac procedures. The drive to meet evolving clinical needs and regulatory standards continues to shape the dynamic and innovation-driven landscape of this market.
Recent Developments
- In October 2022, Medtronic received approval for the expanded label of cardiac lead. These cardiac lead taps into the heart’s natural electrical system and provides patients with needed therapy.
- In October 2022, Merit Medical Systems, Inc., acquired Tempo Lead from BioTrace Medical and expanded its product portfolio in the market.
Market Concentration & Characteristics
The Temporary Cardiac Pacing Wires Market demonstrates moderate to high market concentration, with a few multinational medical device manufacturers holding significant market shares. It is characterized by a focus on clinical reliability, stringent regulatory compliance, and continuous innovation in device design. Leading companies leverage extensive research and development resources to deliver advanced pacing wires with enhanced biocompatibility and infection resistance. Hospitals and cardiac centers seek dependable, high-quality products that meet evolving clinical standards, driving demand for brands with proven track records. Market entry barriers remain substantial due to strict approval processes and high initial investment requirements. Product differentiation centers on safety features, user-friendly designs, and compatibility with modern cardiac procedures. The Temporary Cardiac Pacing Wires Market values partnerships between manufacturers and healthcare providers, supporting ongoing product development and clinician training. It remains dynamic, adapting quickly to technological advances and shifts in global healthcare priorities.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Report Coverage
The research report offers an in-depth analysis based on Product Type, Application, End-User and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market will see steady demand due to the rising prevalence of cardiac arrhythmias and cardiovascular diseases.
- Innovations in biocompatible materials and wire design will enhance patient safety and product reliability.
- Hospitals and cardiac centers will continue to drive adoption through growing interventional cardiology procedures.
- Increased focus on minimally invasive cardiac surgeries will support the use of temporary pacing wires.
- Aging global populations will contribute to a larger patient pool requiring temporary cardiac pacing solutions.
- Regulatory emphasis on product safety and quality will shape manufacturer strategies and investments.
- Emerging economies will experience higher growth rates as healthcare infrastructure and access improve.
- Remote and telemonitoring advancements may influence temporary pacing wire management protocols.
- Market consolidation is likely as leading players pursue mergers and acquisitions to expand portfolios.
- Physician education and training initiatives will further increase procedural success and adoption rates.





