Market Overview
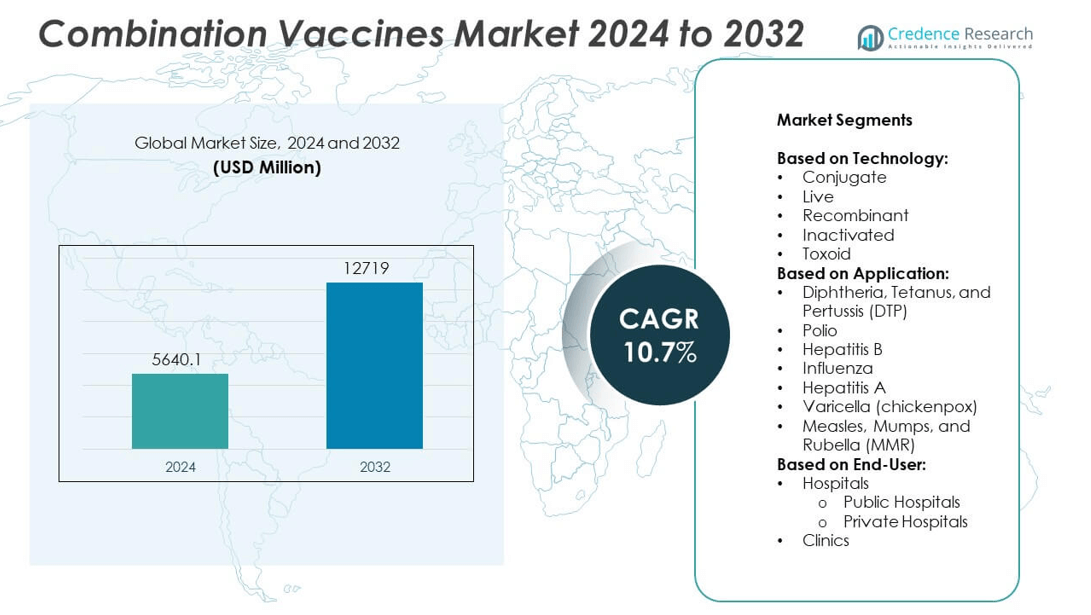
The combination vaccines market size was valued at USD 5640.1 million in 2024 and is anticipated to reach USD 12,719 million by 2032, growing at a compound annual growth rate (CAGR) of 10.7% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Combination Vaccines Market Size 2024 |
USD 5640.1 million |
| Combination Vaccines Market, CAGR |
10.7% |
| Combination Vaccines Market Size 2032 |
USD 12,719 million |
The combination vaccines market grows due to rising infectious disease prevalence and increased government immunization programs worldwide. Advances in vaccine technology improve efficacy and patient compliance, driving demand for multi-antigen formulations. Expanding healthcare infrastructure, especially in emerging markets, supports broader vaccine accessibility. Public awareness campaigns and government funding further boost adoption rates. The market also trends toward innovative delivery methods, such as needle-free systems, enhancing patient experience and safety. Strategic collaborations among pharmaceutical companies accelerate product development and regulatory approvals, fostering rapid market expansion.
The combination vaccines market shows strong growth across North America, Europe, and the Asia Pacific, driven by well-established immunization programs and increasing healthcare investments. Emerging regions like Latin America and the Middle East also present expanding opportunities due to rising public health awareness and government initiatives. Key players such as Sanofi Pasteur, GlaxoSmithKline (GSK), Merck & Co., and Serum Institute of India lead the market through continuous innovation and strategic partnerships. These companies focus on developing advanced multi-antigen vaccines and expanding their global presence to meet growing demand for effective and accessible immunization solutions worldwide.
Market Insights
- The combination vaccines market was valued at USD 5,640.1 million in 2024 and is expected to reach USD 12,719 million by 2032, growing at a CAGR of 10.7% during the forecast period.
- Rising infectious disease prevalence and increased government immunization programs globally drive market growth by improving vaccine coverage and demand.
- Market trends show expanding vaccine portfolios targeting multiple diseases, adoption of innovative delivery technologies like needle-free injectors, and growing focus on emerging markets for broader immunization reach.
- Competitive analysis highlights key players such as Sanofi Pasteur, GlaxoSmithKline, Merck & Co., and Serum Institute of India, who lead through continuous innovation and strategic collaborations.
- Market restraints include stringent regulatory requirements that increase development costs and lengthy approval timelines, limiting rapid product launches and new entrants.
- Geographic analysis indicates strong growth in North America, Europe, and Asia Pacific due to established healthcare infrastructure and government support, with emerging markets gaining traction.
- Challenges like vaccine hesitancy and cold chain logistics in low-resource regions affect market penetration but present opportunities for technological advancements and improved healthcare delivery systems.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers
Rising Prevalence of Infectious Diseases Drives Demand for Combination Vaccines
The increasing incidence of infectious diseases worldwide significantly boosts the combination vaccines market. Governments and healthcare organizations prioritize immunization programs to control outbreaks of diseases such as measles, mumps, rubella, and diphtheria. Combining multiple vaccines into a single injection improves vaccination coverage by reducing the number of shots required, which enhances patient compliance. This streamlined approach helps healthcare providers efficiently manage immunization schedules, especially in pediatric care. It also minimizes the risks associated with multiple injections, improving overall public health outcomes. Growing awareness about vaccine-preventable diseases further supports market expansion.
- For instance, GlaxoSmithKline (GSK) reported delivering over 150 million doses annually of its Infanrix Hexa vaccine, a six-in-one combination vaccine that protects against six diseases including diphtheria and pertussis, thereby significantly improving vaccination adherence globally.
Technological Advancements and Innovation Enhance Vaccine Effectiveness
Innovation in vaccine formulation and delivery methods contributes strongly to market growth. Researchers develop combination vaccines that offer improved efficacy, longer-lasting immunity, and fewer side effects. Novel technologies enable manufacturers to combine multiple antigens without compromising the potency of individual components. This progress reduces the need for booster doses, making immunization programs more cost-effective. The combination vaccines market benefits from ongoing research focused on expanding the range of diseases targeted by combination vaccines. These innovations help address unmet medical needs and widen the acceptance of vaccines globally.
- For instance, Sanofi Pasteur developed the Pentaxim vaccine, which combines five antigens in a single injection, and clinical trials demonstrated a seroprotection rate exceeding 95% for all targeted diseases after the primary vaccination series, showcasing enhanced.
Government Initiatives and Support Encourage Market Expansion
Government policies and funding play a crucial role in driving market growth. Many countries include combination vaccines in their national immunization schedules to streamline vaccination efforts and reduce healthcare costs. Public health campaigns increase awareness and promote vaccine adoption among different population groups. Regulatory approvals for new vaccine combinations facilitate faster market entry for manufacturers. Support from international organizations, such as WHO and UNICEF, further boosts demand in developing regions. It enables improved access to vaccines in underserved areas, creating growth opportunities.
Growing Focus on Pediatric Healthcare and Immunization Coverage
Pediatric healthcare remains a key segment propelling the combination vaccines market. It helps protect children from multiple diseases through a single vaccination visit, simplifying complex immunization regimens. Parents prefer combination vaccines because they reduce discomfort and stress related to multiple injections. Pediatricians recommend these vaccines to improve adherence to vaccination schedules, lowering the risk of disease outbreaks. Increasing birth rates in emerging economies drive demand for effective immunization solutions. The market benefits from growing investments in child health infrastructure and vaccine awareness programs. This focus ensures sustained growth in the coming years.
Market Trends
Expansion of Combination Vaccines Portfolio to Address Diverse Disease Targets
The combination vaccines market experiences a trend toward expanding product portfolios to cover a broader range of diseases. Manufacturers focus on developing multi-antigen vaccines that protect against common childhood illnesses and emerging infectious diseases. This approach allows healthcare providers to offer comprehensive immunization with fewer injections, improving patient convenience and compliance. Companies invest in research to combine vaccines for diseases like influenza, hepatitis, and pneumococcal infections. The trend reflects a shift toward integrated disease prevention strategies. It helps reduce the burden on healthcare systems by streamlining vaccination schedules. Broad-spectrum combination vaccines continue to attract significant attention from both public and private sectors.
- For instance, the Serum Institute of India supplies over 1.5 billion vaccine doses annually to global immunization programs, supporting widespread adoption of combination vaccines in low- and middle-income countries.
Adoption of Advanced Vaccine Delivery Technologies Enhances Market Appeal
The market trends highlight increased adoption of novel vaccine delivery systems that improve patient experience and efficacy. Technologies such as needle-free injectors and microneedle patches gain traction for their potential to reduce pain and simplify administration. Combination vaccines delivered through these innovative methods support mass immunization campaigns and reduce the need for highly trained medical personnel. These advancements also lower the risks of needle-stick injuries and contamination. The market benefits from continuous efforts to make vaccination safer and more accessible globally. It encourages higher uptake among populations with vaccine hesitancy. The adoption of these technologies shapes the future of combination vaccine delivery.
- For instance, Merck & Co.’s Pediarix vaccine, used in over 100 countries, has been administered to millions of children and demonstrated coverage for five key diseases in a single shot, significantly improving pediatric vaccination rates worldwide.
Growing Emphasis on Immunization Programs in Emerging Markets
Emerging markets show an increasing focus on expanding immunization programs, driving growth in the combination vaccines market. Governments and international organizations invest heavily in vaccination infrastructure and awareness campaigns to reduce disease prevalence. Rising healthcare expenditure and improved access to healthcare facilities contribute to higher vaccine adoption rates. The trend includes integration of combination vaccines into national immunization schedules to maximize coverage and efficiency. It reflects a strategic move to control preventable diseases in regions with high population density. Growing demand in these markets presents lucrative opportunities for vaccine manufacturers. The expansion strengthens global health security through broader immunization reach.
Strategic Collaborations and Partnerships Accelerate Market Growth
The combination vaccines market benefits from strategic collaborations between pharmaceutical companies, research institutions, and government bodies. Partnerships accelerate product development and facilitate regulatory approvals across multiple regions. Collaborations allow sharing of technological expertise and resources, reducing time-to-market for new vaccine combinations. The trend also includes licensing agreements and joint ventures aimed at expanding manufacturing capabilities. It enables companies to leverage each other’s strengths to meet growing global demand. This cooperative approach enhances innovation and distribution efficiency. Strategic alliances remain critical for sustaining competitive advantage in the evolving market landscape.
Market Challenges Analysis
Complex Regulatory Requirements and High Development Costs Hinder Market Progress
The combination vaccines market faces significant challenges due to complex regulatory requirements across different regions. Manufacturers must conduct extensive clinical trials to demonstrate the safety and efficacy of multi-antigen formulations, which often prolongs the approval process. Regulatory agencies impose stringent guidelines to ensure product quality and immunogenicity, increasing time and financial investments. High development costs for combination vaccines limit the entry of smaller players and slow down innovation. It also creates barriers to rapid commercialization, delaying patient access to new vaccines. The need to comply with diverse standards in global markets complicates strategic planning for manufacturers. These regulatory and financial hurdles remain critical obstacles to faster market expansion.
Concerns Over Vaccine Hesitancy and Cold Chain Infrastructure Impact Market Adoption
Vaccine hesitancy presents a notable challenge that affects the growth of the combination vaccines market. Misinformation and distrust in vaccines lead to lower immunization rates, especially in certain demographics and regions. It requires sustained public education and awareness programs to improve acceptance and uptake. Furthermore, maintaining cold chain infrastructure to preserve vaccine efficacy poses logistical challenges in low-resource settings. The sensitive nature of combination vaccines demands strict temperature control during storage and transport, increasing operational costs. Limited access to reliable cold chain systems restricts vaccine distribution in remote areas. Overcoming these issues is essential to achieve widespread immunization coverage and market growth.
Market Opportunities
Expanding Immunization Programs and Increasing Government Support Create Significant Growth Opportunities
The combination vaccines market benefits from expanding immunization programs worldwide, driven by government initiatives to improve public health. Many countries incorporate combination vaccines into national vaccination schedules to enhance coverage and simplify administration. Increased funding and policy support encourage vaccine adoption, particularly in emerging economies with large pediatric populations. It enables manufacturers to access new markets and scale production efficiently. Government partnerships with international organizations further strengthen distribution networks and improve vaccine accessibility. These factors create a favorable environment for market expansion and innovation. The growing focus on preventive healthcare drives sustained demand for combination vaccines.
Advancements in Vaccine Technology and Development of Novel Formulations Offer Market Potential
The market presents opportunities through advancements in vaccine technology, including the development of next-generation combination vaccines targeting a wider range of diseases. Innovations in antigen formulation and delivery methods allow for improved efficacy and patient compliance. It opens doors for introducing vaccines that address unmet medical needs and emerging infectious threats. The combination vaccines market can leverage these technological breakthroughs to create more effective and safer products. Collaborations between research institutions and pharmaceutical companies accelerate development pipelines. Expanding vaccine portfolios with novel combinations enhances competitive positioning and market reach. These opportunities support long-term growth and industry evolution.
Market Segmentation Analysis:
By Technology:
Conjugate vaccines hold a significant share due to their ability to generate strong immune responses by linking antigens to carrier proteins. Live vaccines follow closely, favored for their effectiveness in inducing long-lasting immunity. Recombinant vaccines gain traction with advances in genetic engineering, offering targeted protection with fewer side effects. Inactivated vaccines remain widely used for their safety profile, particularly in populations with weakened immune systems. Toxoid vaccines address bacterial infections by neutralizing toxins, maintaining steady demand in immunization programs. Each technology type supports diverse vaccine combinations, broadening the market’s reach.
- For instance, Serum Institute of India to produce up to an additional 100 million COVID-19 vaccine doses for India and low-and middle-income countries
By Application:
The combination vaccines market focuses on several critical infectious diseases. The diphtheria, tetanus, and pertussis (DTP) segment dominates due to the inclusion of these vaccines in childhood immunization schedules worldwide. Polio vaccines sustain demand amid global eradication efforts. Hepatitis B and influenza vaccines witness growth driven by increasing awareness and vaccination campaigns. Hepatitis A and varicella (chickenpox) vaccines also contribute to market expansion by targeting prevalent viral infections. The measles, mumps, and rubella (MMR) vaccine segment remains vital in preventing outbreaks, supported by ongoing public health initiatives. These applications reflect the market’s emphasis on preventing high-impact diseases through effective combination immunizations.
- For instance, Merck & Co. reported that over 200 million doses of its MMR-II vaccine have been distributed globally, significantly contributing to measles and rubella control efforts.
By End-User:
The combination vaccines market includes hospitals and clinics, with hospitals further divided into public and private institutions. Public hospitals serve a large patient base, especially in government-led immunization programs, driving substantial vaccine demand. Private hospitals contribute by catering to populations seeking personalized healthcare services, often opting for the latest vaccine combinations. Clinics, including specialized and community health centers, play a crucial role in improving vaccine accessibility, particularly in urban and semi-urban areas. The distribution across various healthcare settings ensures broad vaccine reach, supporting sustained market growth and improved public health outcomes.
Segments:
Based on Technology:
- Conjugate
- Live
- Recombinant
- Inactivated
- Toxoid
Based on Application:
- Diphtheria, Tetanus, and Pertussis (DTP)
- Polio
- Hepatitis B
- Influenza
- Hepatitis A
- Varicella (chickenpox)
- Measles, Mumps, and Rubella (MMR)
Based on End-User:
- Hospitals
- Public Hospitals
- Private Hospitals
- Clinics
Based on the Geography:
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis
North America
North America leads the market with approximately 32% share in 2024, driven by advanced healthcare systems, strong research and development activities, and high vaccine adoption rates. The presence of major pharmaceutical companies and well-established immunization programs supports steady growth in this region. Increasing investments in vaccine technology and awareness campaigns contribute to the rising demand for combination vaccines in both pediatric and adult populations. North America’s regulatory environment, though stringent, facilitates innovation and market entry for novel vaccines, reinforcing its market dominance.
Europe
Europe accounts for around 27% of the combination vaccines market share. This region benefits from comprehensive vaccination policies and widespread public health programs across countries like Germany, France, and the United Kingdom. The European Union’s emphasis on preventive healthcare and funding for vaccine research accelerates product development and accessibility. Europe also sees growing adoption of combination vaccines in response to increased awareness about vaccine-preventable diseases and aging populations requiring immunization. Stringent regulatory frameworks ensure high standards for vaccine safety and efficacy, encouraging public trust and uptake. The region’s established cold chain infrastructure enhances efficient vaccine distribution, supporting continuous market growth.
The Asia Pacific
The Asia Pacific region holds approximately 25% market share, representing a fast-growing segment due to rising population, increasing healthcare expenditure, and expanding immunization initiatives in countries such as China, India, and Japan. The combination vaccines market in Asia Pacific benefits from government efforts to reduce disease burden through mass vaccination programs. Rapid urbanization and improvements in healthcare access further increase vaccine demand. Manufacturers focus on tailoring vaccine formulations suitable for local epidemiology, driving innovation in the region. However, challenges such as cold chain logistics and vaccine hesitancy remain, though ongoing investments aim to address these issues and support market expansion.
Latin America
Latin America captures around 10% of the market share, driven by growing public health awareness and efforts to enhance immunization coverage in countries like Brazil and Mexico. The region benefits from collaborations with international organizations to improve vaccine accessibility and affordability. Increasing healthcare infrastructure and government subsidies for vaccines encourage adoption of combination vaccines. However, economic disparities and logistical challenges still limit full market potential. Continuous improvement in healthcare delivery and vaccination campaigns promise sustained growth in Latin America.
The Middle East and Africa
The Middle East and Africa account for the remaining 6% of the combination vaccines market share. These regions face challenges such as limited healthcare infrastructure, political instability, and vaccine accessibility issues, which restrict market growth. Nonetheless, ongoing initiatives supported by global health organizations and government programs aim to improve immunization rates. Investments in cold chain systems and awareness campaigns contribute to gradual market development. The growing focus on preventive healthcare in these regions presents future opportunities despite current hurdles. Overall, the regional dynamics shape the global market’s trajectory, with each region contributing uniquely to the combination vaccines market growth.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Novavax
- Serum Institute of India
- Merck & Co., Inc.
- Beijing Minhai Biological Technology Co., Ltd.
- Sanofi Pasteur
- Sinovac Biotech
- Panacea Biotec
- GlaxoSmithKline (GSK)
- AstraZeneca
- Johnson & Johnson
Competitive Analysis
Key players in the combination vaccines market include Sanofi Pasteur, GlaxoSmithKline (GSK), Merck & Co., Serum Institute of India, Novavax, Sinovac Biotech, Panacea Biotec, AstraZeneca, Johnson & Johnson, and Beijing Minhai Biological Technology Co., Ltd. These companies dominate the market through extensive research and development efforts, focusing on innovative multi-antigen vaccines that improve efficacy and patient compliance. They invest heavily in advanced technologies to develop safer and more effective formulations, enabling them to maintain competitive advantages. Strategic collaborations, partnerships, and acquisitions help expand their product portfolios and global reach. Leading players leverage strong distribution networks to ensure widespread vaccine accessibility, especially in emerging markets. Their ability to navigate complex regulatory environments efficiently accelerates time-to-market for new products. Additionally, ongoing investments in manufacturing capacity enhance production scalability to meet growing demand. Competitive pricing strategies and targeted marketing campaigns further strengthen their market positions. These companies continuously focus on improving vaccine delivery methods to address patient preferences and reduce administration challenges. By prioritizing innovation and global expansion, these key players sustain their leadership and drive growth within the combination vaccines market.
Recent Developments
- In June 2025, the Central Drugs Standard Control Organization (CDSCO) asked Serum Institute of India to revise the protocol for its Phase II/III clinical trial for a Trivalent Nanoparticle Influenza Vaccine and COVID-Influenza Combination (CIC) Vaccine. The revised protocol includes a four-arm study design, increased sample size, and additional immunogenicity analysis.
- In June 2025, SIIPL became the first vaccine manufacturer to submit a Prequalification Dossier (PQD) for a vaccine in the electronic Common Technical Document (eCTD) format to the WHO.
- In May 2024, Novavax collaboration with Sanofi with an upfront payment of $500 million to develop new flu-COVID-19 combination vaccines.
Market Concentration & Characteristics
The combination vaccines market exhibits a moderately concentrated structure, dominated by a few leading pharmaceutical companies that hold significant market shares. These key players invest heavily in research and development to innovate multi-antigen vaccines that address a broad spectrum of infectious diseases. The market’s high entry barriers stem from stringent regulatory requirements, substantial development costs, and the need for advanced manufacturing capabilities. It demands strong expertise in vaccine formulation and delivery technologies, limiting the presence of smaller companies. The market also features strategic collaborations and partnerships that enhance product pipelines and expand geographic reach. It maintains a dynamic competitive environment where innovation, regulatory compliance, and efficient supply chain management drive success. The combination vaccines market benefits from growing demand in both developed and emerging economies, prompting manufacturers to tailor their products to regional disease profiles and healthcare infrastructure. It emphasizes improving immunization coverage through convenient, cost-effective solutions that reduce the number of injections and improve patient compliance. This focus shapes market characteristics by balancing innovation with accessibility and affordability, ensuring sustained growth in global vaccination programs.
Report Coverage
The research report offers an in-depth analysis based on Technology, Application, End-User and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The combination vaccines market will continue to grow steadily due to increasing demand for multi-disease protection.
- Advances in vaccine technology will lead to the development of more effective and safer combination vaccines.
- Expansion of immunization programs in emerging markets will drive higher adoption rates globally.
- Manufacturers will focus on creating vaccines targeting a wider range of infectious diseases.
- Strategic partnerships and collaborations will accelerate product innovation and market penetration.
- Regulatory frameworks will evolve to support faster approval of combination vaccines without compromising safety.
- Improved vaccine delivery methods will enhance patient compliance and reduce administration challenges.
- Growing public awareness and education campaigns will reduce vaccine hesitancy in various regions.
- Investments in cold chain infrastructure will improve vaccine accessibility in remote and low-resource areas.
- The market will witness increased competition, encouraging companies to innovate and optimize production efficiency.




