Market Overview
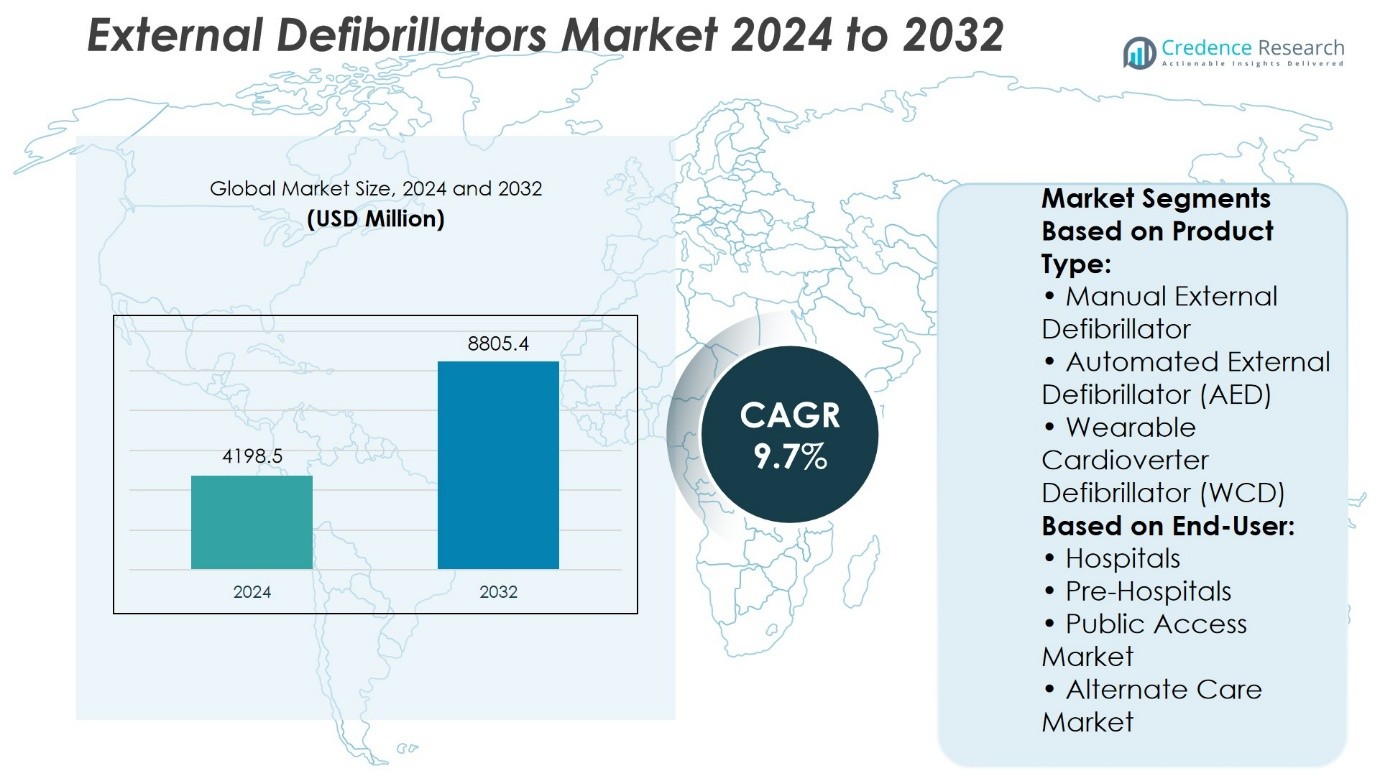
External Defibrillators Market size was valued at USD 4198.5 million in 2024 and is anticipated to reach USD 8805.4 million by 2032, at a CAGR of 9.7% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2024 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| External Defibrillators Market Size 2024 |
USD 4198.5 Million |
| External Defibrillators Market, CAGR |
9.7% |
| External Defibrillators Market Size 2032 |
USD 8805.4 Million |
The External Defibrillators Market grows driven by the rising prevalence of cardiovascular diseases and sudden cardiac arrests worldwide. It benefits from technological advancements that enhance device portability, accuracy, and user-friendliness, including real-time monitoring and connectivity features. Government initiatives promoting public access defibrillation programs and mandatory AED installations in public spaces expand market reach. Increasing consumer awareness encourages adoption in homes and alternate care settings. Trends show a shift toward smart, connected devices with improved battery life and compact designs. Training programs further empower non-professional users, boosting effective utilization and overall market growth.
North America leads the External Defibrillators Market with a 35% share, driven by advanced healthcare infrastructure and strong regulatory support. Europe follows with 28%, supported by government mandates and high consumer awareness. Asia-Pacific shows rapid growth due to rising cardiac disease prevalence and healthcare investments. Key players dominating the market include Koninklijke Philips N.V., Stryker, ZOLL Medical Corporation, Nihon Kohden Corporation, and Progetti Srl. These companies focus on innovation, expanding distribution, and strategic partnerships to strengthen their global presence.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The External Defibrillators Market was valued at USD 4,198.5 million in 2024 and is expected to reach USD 8,805.4 million by 2032, growing at a CAGR of 9.7%.
- Rising prevalence of cardiovascular diseases and sudden cardiac arrests drives demand for external defibrillators globally.
- Technological advancements improve device portability, accuracy, and connectivity, enhancing user experience and emergency response.
- Government initiatives promote public access defibrillation programs and mandate AED installations in public areas, expanding market penetration.
- High costs and maintenance requirements pose challenges for wider adoption, especially in low-resource settings.
- North America leads with a 35% market share, followed by Europe at 28%, and Asia-Pacific showing rapid growth due to healthcare investments.
- Key players such as Koninklijke Philips N.V., Stryker, and ZOLL Medical Corporation focus on innovation and global expansion to maintain competitive advantage.
Market Drivers
Rising Incidence of Cardiovascular Diseases Driving Demand for External Defibrillators
The growing prevalence of cardiovascular diseases globally significantly fuels the External Defibrillators Market. Increased cases of sudden cardiac arrests create urgent demand for rapid response devices capable of restoring normal heart rhythm. It enables timely intervention in emergency situations, improving survival rates and reducing long-term complications. Healthcare providers and public safety organizations prioritize equipping ambulances, hospitals, and public spaces with these devices. Growing awareness about cardiac emergencies also encourages individuals and institutions to adopt external defibrillators. This trend drives manufacturers to focus on enhancing device accessibility and ease of use.
- For instance, ZOLL Medical Corporation reports that its wearable LifeVest external defibrillator has been prescribed to over 200,000 patients worldwide as of 2021, and in clinical trials.
Technological Advancements Enhancing Functionality and User Experience in External Defibrillators
Technological innovation remains a critical driver for market expansion by improving device accuracy, portability, and user interface. Integration of features like voice prompts, real-time ECG monitoring, and connectivity to emergency services increases device effectiveness. It reduces hesitation among bystanders to operate defibrillators during cardiac emergencies. Manufacturers invest in research to develop compact and lightweight designs that enable easy transportation and quick deployment. Enhanced battery life and automatic self-testing capabilities also increase reliability. These advancements expand the applicability of external defibrillators beyond hospitals to homes and public venues.
- For instance, Philips reports that over 1 million HeartStart OnSite (HS1) units have been deployed worldwide, and its Quick Shock feature delivers a therapeutic shock typically within 8 seconds after the end of a CPR interval.
Government Initiatives and Regulatory Support Promoting Wider Adoption of External Defibrillators
Supportive policies and funding from governments and health organizations accelerate market growth by promoting public access defibrillation programs. Many countries mandate installation of external defibrillators in high-traffic areas such as airports, schools, and sports arenas. Training campaigns and certification programs increase community readiness to respond to cardiac arrests. It creates opportunities for manufacturers to supply large volumes of devices for public use. Regulatory approvals streamline product launches, ensuring devices meet safety and performance standards. These measures collectively boost market penetration and awareness.
Increasing Consumer Awareness and Preference for Home-Based External Defibrillators
Rising health consciousness among consumers drives demand for external defibrillators designed for personal use. People seek devices that offer immediate cardiac emergency support within residential settings, where most cardiac events occur. It encourages companies to develop user-friendly models with simplified interfaces and clear instructions. Enhanced focus on preventive healthcare fosters adoption of home-based medical devices, including external defibrillators. The availability of portable and automated versions supports non-medical users in emergency scenarios. This shift widens the market beyond professional healthcare environments.
Market Trends
Growing Integration of Smart Technologies and Connectivity in External Defibrillators
The External Defibrillators Market experiences a shift toward devices equipped with advanced smart features and connectivity options. Manufacturers incorporate Bluetooth and Wi-Fi to enable remote monitoring and data transmission to healthcare providers. It facilitates real-time tracking of device status and patient information, improving emergency response coordination. Enhanced software algorithms support more accurate arrhythmia detection and personalized shock delivery. This trend improves device reliability and user confidence during cardiac emergencies. It also enables integration into broader health management systems, expanding device functionality beyond standalone use.
- For instance, Philips has shipped 1.5 million AED units featuring technologies such as Bluetooth‑enabled data transfer and Wi‑Fi monitoring capabilities.
Expansion of Public Access Defibrillation Programs Increasing Market Reach
Public access to external defibrillators gains momentum globally, driven by government policies and community health initiatives. It leads to increased installation of automated external defibrillators (AEDs) in public spaces, workplaces, and educational institutions. Training programs empower laypersons to use defibrillators effectively during cardiac arrests. The market responds by developing devices with simplified operation and clear voice prompts to assist non-medical users. Wider availability of defibrillators outside traditional healthcare settings enhances survival rates and emergency preparedness. This trend creates sustained demand for portable and user-friendly external defibrillators.
- For instance, Japan’s public access defibrillation efforts saw the cumulative number of public‑access AEDs rise to 203,924 units.
Rising Demand for Portable and Lightweight External Defibrillators Enhancing Market Growth
Portability and ease of use remain key factors influencing purchasing decisions in the External Defibrillators Market. Manufacturers prioritize compact and lightweight designs to improve device transport and accessibility in emergencies. It allows rapid deployment by first responders and bystanders in diverse environments. Battery technology advances extend operational life and reduce maintenance frequency, supporting device readiness. Increased focus on ergonomic designs also improves user comfort during handling. This trend aligns with growing adoption of external defibrillators in home care and remote locations, broadening market applications.
Emphasis on Training and Education to Improve Defibrillator Usage and Outcomes
Training initiatives gain importance to maximize the effectiveness of external defibrillators during cardiac events. Healthcare organizations and governments invest in widespread CPR and AED training programs. It increases confidence among non-professional users, reducing hesitation and misuse. Simulated training devices and digital platforms support skill retention and accessibility. Manufacturers collaborate with training providers to ensure device operation aligns with educational content. Enhanced user competence directly contributes to improved patient survival and device adoption. This trend drives demand for defibrillators with intuitive interfaces and guided instructions.
Market Challenges Analysis
High Cost and Maintenance Requirements Limit Wider Adoption of External Defibrillators
The External Defibrillators Market faces challenges due to the relatively high purchase and maintenance costs of devices. Initial investment in advanced defibrillators can be prohibitive for smaller healthcare facilities and public institutions. It requires regular servicing, battery replacement, and electrode pad renewal to maintain operational readiness. These ongoing expenses discourage potential buyers from investing in multiple units, especially in low-resource settings. Budget constraints often delay device upgrades or replacements, impacting overall accessibility. Cost concerns also affect consumer willingness to purchase home-use models, limiting market penetration.
Lack of Awareness and Training Hampers Effective Utilization of External Defibrillators
Insufficient awareness about cardiac emergencies and defibrillator operation remains a significant barrier in the External Defibrillators Market. Many potential users hesitate due to unfamiliarity with device functions or fear of misuse during critical moments. It underscores the need for widespread education and hands-on training programs to build user confidence. Limited availability of certified training restricts effective community response, particularly in remote or underserved regions. Without proper knowledge, the benefits of increased defibrillator deployment cannot be fully realized. This challenge calls for coordinated efforts among manufacturers, healthcare providers, and policymakers to improve accessibility and training.
Market Opportunities
Expansion into Emerging Markets Presents Significant Growth Potential for External Defibrillators
Emerging economies offer vast opportunities for the External Defibrillators Market due to rising healthcare investments and increasing cardiovascular disease prevalence. It enables manufacturers to target underserved regions with tailored products suited for varying infrastructure and budget constraints. Government initiatives in these markets often focus on improving emergency response capabilities, supporting wider device adoption. The growing urbanization and improving healthcare infrastructure create demand for advanced medical technologies, including external defibrillators. Collaborations with local distributors and healthcare providers can facilitate market entry and expansion. This environment encourages innovation in cost-effective, portable devices to meet regional needs.
Integration of Artificial Intelligence and Remote Monitoring Technologies Opens New Frontiers in Market Development
Technological advancements such as artificial intelligence (AI) and remote monitoring offer promising opportunities in the External Defibrillators Market. AI algorithms enhance arrhythmia detection accuracy and optimize shock delivery, improving patient outcomes. It allows predictive maintenance by monitoring device status in real time, reducing downtime and operational risks. Remote monitoring enables healthcare professionals to track usage data and intervene promptly when necessary. Manufacturers who integrate these technologies can differentiate their offerings and create value-added services. Growing interest in connected healthcare devices supports the adoption of smart external defibrillators in hospitals and home care settings. This trend fosters continuous innovation and expands market applications.
Market Segmentation Analysis:
By Product Type
The External Defibrillators Market segments primarily into Manual External Defibrillators, Automated External Defibrillators (AED), and Wearable Cardioverter Defibrillators (WCD). Manual External Defibrillators remain essential in hospital and clinical settings, where trained professionals deliver precise energy doses during cardiac emergencies. It offers control and flexibility for managing complex arrhythmia cases. The Automated External Defibrillator segment experiences rapid growth due to ease of use and broad application in public and pre-hospital environments. AEDs feature user-friendly interfaces and voice prompts that guide laypersons through emergency procedures, improving survival rates outside medical facilities. Wearable Cardioverter Defibrillators cater to patients at high risk of sudden cardiac arrest, providing continuous monitoring and automatic shock delivery while allowing patient mobility.
- For instance, ZOLL Medical Corporation LifeVest WCD has been prescribed to more than 200,000 patients worldwide.
By End User
The market divides by end user into hospitals, pre-hospitals, public access, and alternate care markets. Hospitals remain the largest consumers due to high patient volume and the need for advanced cardiac care equipment. It requires diverse defibrillator types to address various clinical scenarios, including emergency rooms and cardiac units. The pre-hospital segment includes ambulances and emergency medical services that demand portable and rapid-response devices. It plays a critical role in reducing response times during out-of-hospital cardiac arrests. Public access markets focus on widespread deployment of AEDs in airports, schools, workplaces, and other public locations to empower bystanders in emergencies. Regulatory mandates and increasing awareness drive growth in this segment. The alternate care market includes nursing homes, rehabilitation centers, and home care settings, where external defibrillators enhance patient safety by providing emergency solutions beyond hospital environments.
- For instance, Lutheran General Hospital had deployed a total of 82 manual and semi‑automated Philips defibrillators on their campus, consisting of 18 CodeMaster XL+ units and 64 43100 Series units.
Segments:
Based on Product Type:
- Manual External Defibrillator
- Automated External Defibrillator (AED)
- Wearable Cardioverter Defibrillator (WCD)
Based on End-User:
- Hospitals
- Pre-Hospitals
- Public Access Market
- Alternate Care Market
Based on the Geography:
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis
North America
North America holds the largest share of the External Defibrillators Market, accounting for approximately 35% of the global market. The region benefits from a well-established healthcare infrastructure, widespread awareness of sudden cardiac arrest (SCA), and proactive government initiatives promoting public access defibrillation programs. High adoption rates of automated external defibrillators (AEDs) in public spaces, workplaces, and residential settings support market growth. It also benefits from significant investments in research and development by key market players headquartered in the U.S. and Canada. Increasing training programs and favorable reimbursement policies further boost device adoption.
Europe
Europe represents the second-largest region, capturing roughly 28% of the market share. This growth results from increasing cardiovascular disease incidence and strong government support for deploying external defibrillators in public areas. Regulatory frameworks mandate AED installation in airports, sports venues, and educational institutions, expanding market penetration. Countries such as Germany, the UK, and France drive demand due to improved emergency medical services and high consumer awareness. It sees consistent growth in both hospital and pre-hospital segments, with technological advancements enhancing device features tailored to European healthcare standards.
Asia-Pacific
The Asia-Pacific region commands about 22% of the External Defibrillators Market and shows rapid expansion due to rising healthcare expenditure and increasing incidence of cardiac diseases. Growing urbanization, improved healthcare infrastructure, and rising awareness contribute to rising adoption rates in countries such as China, Japan, India, and Australia. It witnesses expanding government initiatives focusing on emergency medical response enhancement and increased availability of training programs. The region also sees rising investments by international manufacturers establishing local production and distribution networks, improving accessibility and affordability.
Latin America
Latin America holds a market share of approximately 8%, driven by gradual improvements in healthcare infrastructure and growing awareness of cardiovascular health risks. Brazil and Mexico emerge as key contributors due to rising investments in public health and emergency services. It faces challenges related to affordability and accessibility in rural areas but benefits from government initiatives aiming to improve public health education and emergency preparedness. The market experiences steady growth through increased AED installations in public and private sectors.
Middle East and Africa
The Middle East and Africa account for around 7% of the market share. The region demonstrates growing demand driven by rising incidences of cardiovascular diseases and increasing healthcare investments. Countries such as the UAE, Saudi Arabia, and South Africa lead market growth with expanding hospital networks and enhanced emergency response capabilities. It benefits from government programs promoting public health awareness and AED accessibility. However, challenges related to economic disparities and infrastructural gaps remain, limiting broader penetration. Continued focus on improving healthcare delivery and public access initiatives supports future market development.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- MS Westfalia GmbH
- Progetti Srl
- Koninklijke Philips N.V.
- Bexen Cardio
- Silverline Meditech Pvt. Ltd.
- Nihon Kohden Corporation
- AMI Italia
- ZOLL Medical Corporation
- Schiller AG
- Stryker
Competitive Analysis
The External Defibrillators Market features strong competition among leading players such as Koninklijke Philips N.V., Stryker, ZOLL Medical Corporation, Nihon Kohden Corporation, Progetti Srl, Schiller AG, MS Westfalia GmbH, AMI Italia, Bexen Cardio, and Silverline Meditech Pvt. Ltd. The External Defibrillators Market remains highly competitive, driven by continuous innovation in device technology and expanding applications across healthcare and public sectors. Companies focus on enhancing device portability, ease of use, and integration with smart technologies to improve emergency response effectiveness. Increasing investments in research and development lead to improvements in battery life, real-time monitoring, and automated operation features. Market players also strengthen their position by expanding global distribution networks and forming strategic partnerships with healthcare providers and governments. The growing emphasis on public access defibrillation programs and training initiatives further intensifies competition, encouraging manufacturers to deliver reliable and cost-effective solutions. This dynamic environment fosters rapid technological advancement and broader adoption of external defibrillators worldwide.
Recent Developments
- In July 2025, Progetti launched a new division called Defib Supplies in partnership with Intermedical (UK) Ltd to expand defibrillator access in the UK.
- In November 2024, Asahi Kasei Electronics Corporation announced its participation in CES® 2025, where it plans to unveil advancements in health technology, including vital sensing and elderly monitoring solutions.
- In January 2024, Element Science received a CE mark in Europe and approval in the U.K. for its patch-based cardioverter defibrillator, designed to offer a more wearable option for individuals at risk of sudden cardiac arrest who may not qualify for or prefer not to have a long-term implant.
- In October 2023, Medtronic plc received FDA approval for its Aurora EV-ICD™ MRI SureScan™ Extravascular Implantable Cardioverter-Defibrillator and Epsila EV™ MRI SureScan™ defibrillation lead.
Market Concentration & Characteristics
The External Defibrillators Market exhibits a moderately concentrated structure, dominated by a few key players who hold significant market shares due to their strong brand presence, extensive product portfolios, and advanced technological capabilities. It features a competitive landscape where innovation and strategic collaborations drive market dynamics. Leading companies invest heavily in research and development to introduce devices with improved accuracy, user-friendliness, and connectivity features. The market balances between high-end automated external defibrillators designed for professional use and more accessible, simplified models aimed at public access and home care segments. Regional diversity influences market characteristics, with developed regions emphasizing advanced features and emerging markets focusing on affordability and accessibility. It faces challenges such as regulatory compliance and high production costs, which create barriers for new entrants, thereby maintaining the dominance of established players. Despite this, smaller and regional manufacturers compete by targeting niche segments and offering cost-effective solutions tailored to local needs. The External Defibrillators Market’s characteristics include rapid technological advancement, increasing adoption in non-traditional settings, and growing public awareness about sudden cardiac arrest. This environment encourages continuous product enhancements and expanded distribution channels, contributing to steady market growth and evolving competitive strategies.
Report Coverage
The research report offers an in-depth analysis based on Product Type, End-User and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The External Defibrillators Market will expand due to increasing prevalence of cardiovascular diseases globally.
- Advanced features such as AI-based arrhythmia detection will improve device accuracy and patient outcomes.
- Growing government initiatives will drive wider public access defibrillator deployment in urban and rural areas.
- Portable and lightweight designs will become standard, enhancing device usability in emergency situations.
- Integration with mobile applications will enable real-time monitoring and remote device management.
- Training programs for non-professional users will increase, boosting device adoption outside clinical settings.
- Emerging markets will present significant growth opportunities due to rising healthcare investments.
- Demand for wearable cardioverter defibrillators will increase among patients with high cardiac risk.
- Regulatory support will streamline approvals, accelerating product launches and innovations.
- Collaboration between technology firms and healthcare providers will foster development of connected defibrillation solutions.




