Market Overview
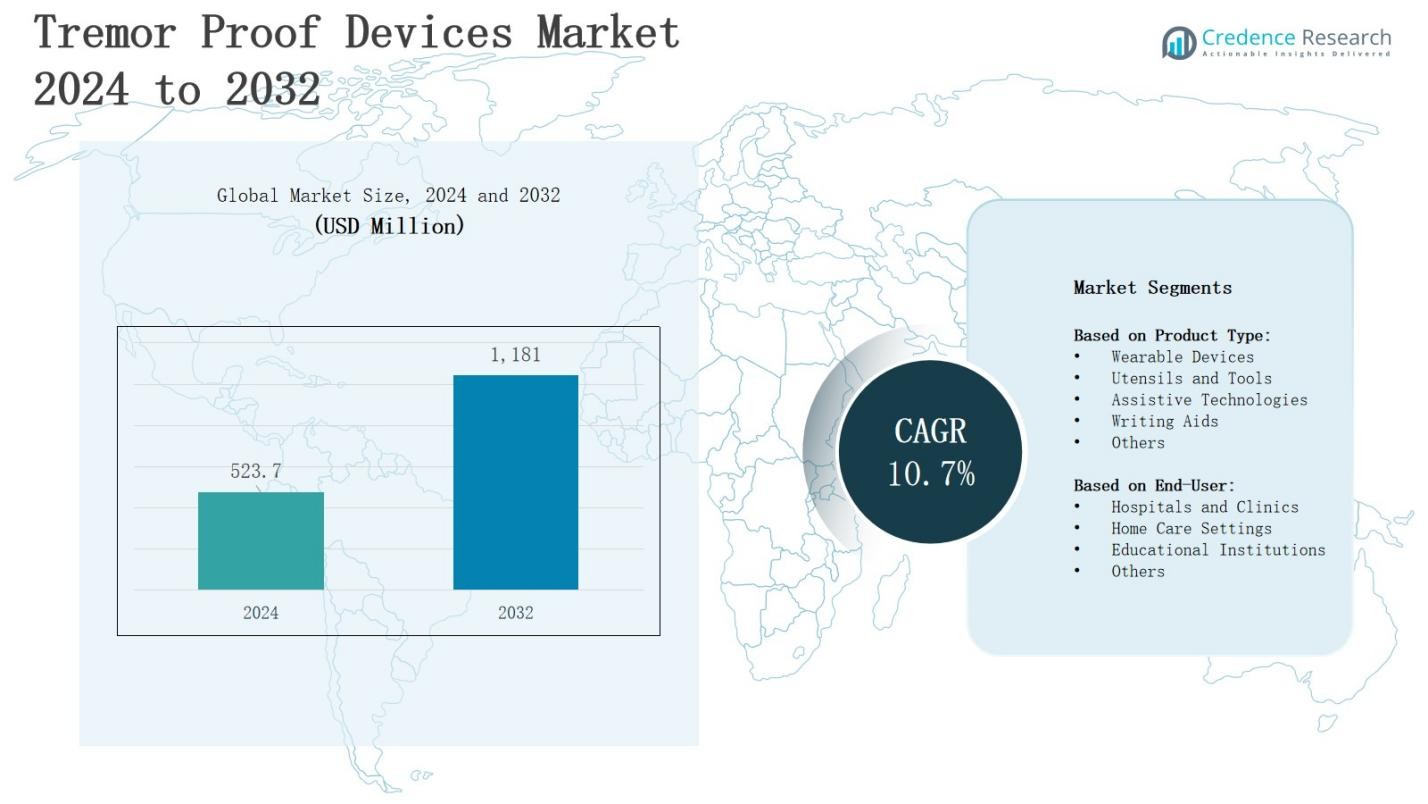
The global tremor proof devices market is projected to grow from USD 523.7 million in 2024 to USD 1,181 million by 2032, registering a CAGR of 10.7% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2024 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Tremor Proof Devices Market Size 2024 |
USD 523.7 Million |
| Tremor Proof Devices Market, CAGR |
10.7% |
| Tremor Proof Devices Market Size 2032 |
USD 1,181 Million |
The tremor proof devices market grows due to rising prevalence of neurological disorders such as Parkinson’s disease, essential tremor, and multiple sclerosis, coupled with increasing demand for assistive technologies that enhance patient independence and quality of life. Advancements in sensor technology, AI-driven motion stabilization, and lightweight ergonomic designs are driving adoption across healthcare and personal use segments. Growing awareness, supportive reimbursement policies, and expanding elderly populations further strengthen market potential. Trends include integration with wearable health monitoring systems, telemedicine compatibility, and customized solutions for varying tremor severity, enabling broader accessibility and improved patient compliance.
The tremor proof devices market spans North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa, each contributing to global growth through distinct adoption drivers. North America leads with advanced healthcare infrastructure, followed by Europe with strong quality-of-life focus and Asia-Pacific with expanding access and affordability. Latin America and the Middle East & Africa show emerging potential amid healthcare modernization. Key players include Steadiwear Inc., Medtronic plc, GyroGear Ltd., Bioness Inc., Lift Labs, Boston Scientific Corporation, NEOFECT, Abbott Laboratories, Penumbra, Inc., and STMicroelectronics N.V.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The global tremor proof devices market is projected to grow from USD 523.7 million in 2024 to USD 1,181 million by 2032, registering a CAGR of 10.7% during the forecast period.
- Rising prevalence of neurological disorders such as Parkinson’s disease, essential tremor, and multiple sclerosis drives demand, supported by an expanding elderly population and higher diagnosis rates.
- Advancements in AI-driven motion stabilization, sensor-based feedback systems, and ergonomic designs enhance usability and boost adoption across healthcare and personal use segments.
- Supportive healthcare policies, reimbursement programs, and public awareness campaigns increase affordability and encourage wider adoption in developed markets.
- High costs, inconsistent insurance coverage, and limited access in rural and low-income regions remain key challenges alongside stringent regulatory requirements and lack of standardization.
- North America leads with 38% share, followed by Europe at 27%, Asia-Pacific at 24%, Latin America at 6%, and the Middle East & Africa at 5%.
- Key players include Steadiwear Inc., Medtronic plc, GyroGear Ltd., Bioness Inc., Lift Labs (Verily Life Sciences LLC), Boston Scientific Corporation, NEOFECT, Abbott Laboratories, Penumbra, Inc., and STMicroelectronics N.V.
Market Drivers
Rising Prevalence of Neurological Disorders
The tremor proof devices market expands with the increasing incidence of neurological conditions such as Parkinson’s disease, essential tremor, and multiple sclerosis. It addresses the need for effective solutions that reduce hand tremors and improve daily functioning. Aging populations worldwide contribute significantly to demand, as tremor disorders are more common among older adults. The market benefits from growing diagnosis rates, enhanced awareness of treatment options, and medical community endorsements, driving steady adoption.
- For instance, the Motus Nova company developed the MOTIMOVE device, which uses electrical muscle stimulation to increase limb stiffness and reduce tremors, achieving clinically significant tremor suppression in patients with essential tremor and Parkinson’s disease.
Advancements in Assistive Technology
The tremor proof devices market benefits from rapid technological progress in motion stabilization, precision engineering, and sensor-based feedback systems. It leverages AI algorithms to adapt to individual tremor patterns, improving device accuracy and comfort. Lightweight, ergonomic designs increase usability for long-term wear. Manufacturers invest in R&D to develop multi-functional devices compatible with existing mobility aids. Enhanced battery life and wireless connectivity further expand usage scenarios, strengthening market competitiveness and user satisfaction.
- The Liftware Steady spoon, designed for essential tremor users, employs motion-sensing technology to counteract tremors during eating tasks. Clinical studies have shown it can suppress tremors by about 73%, improving users’ ability to eat independently.
Supportive Healthcare Policies and Reimbursement Programs
The tremor proof devices market gains momentum from government initiatives and healthcare policies that support assistive technology access. It benefits from insurance coverage, subsidies, and tax incentives in developed markets, making devices more affordable for patients. Hospitals and rehabilitation centers integrate these solutions into therapy programs, enhancing treatment outcomes. Public health campaigns emphasize the role of assistive devices in preserving independence, fostering a positive perception that encourages higher adoption rates globally.
Growing Integration with Digital Health Ecosystems
The tremor proof devices market increasingly aligns with the expansion of digital health platforms and telemedicine. It offers compatibility with wearable health monitors, enabling remote symptom tracking and data sharing with healthcare providers. This integration supports personalized therapy adjustments and early intervention strategies. Demand rises as patients and caregivers seek connected solutions that combine tremor management with broader health monitoring. These capabilities position the market for strong long-term growth opportunities worldwide.
Market Trends
Integration of Artificial Intelligence and Smart Sensors
The tremor proof devices market is witnessing increased adoption of AI-driven algorithms and advanced sensor technology to enhance tremor detection and compensation accuracy. It enables real-time adjustments tailored to individual movement patterns, improving usability and comfort. Devices now feature adaptive learning capabilities that refine performance over time. Manufacturers focus on embedding multi-axis gyroscopes, accelerometers, and precision motors to achieve seamless motion stabilization. This trend enhances device efficiency, user confidence, and long-term adherence to treatment.
- For instance, the Felix NeuroAI Wristband is an AI-powered device cleared by the FDA that reduces tremor-related functional limitations by delivering personalized, adaptive stimulation throughout the day based on real-time data, improving daily task performance without surgery or drugs.
Expansion of Wearable and Ergonomic Designs
The tremor proof devices market is shifting toward lightweight, discreet, and ergonomically designed products that improve comfort and encourage daily use. It emphasizes aesthetics and portability to cater to both medical and lifestyle applications. Adjustable fittings and customizable grip mechanisms address diverse patient needs. Wearables are becoming more discreet, blending with everyday accessories like watches or bracelets. This design evolution increases acceptance among younger demographics and supports continuous use without social discomfort or fatigue.
- For instance, the GyroGlove™ uses a rapidly spinning gyroscope to stabilize the hand against tremors while maintaining a wearable form factor, although it is somewhat bulky and expensive.
Integration with Telemedicine and Remote Monitoring
The tremor proof devices market aligns with the growth of telehealth solutions, enabling remote monitoring and therapy adjustments. It supports data transmission to healthcare providers for real-time performance tracking and symptom analysis. This integration allows early detection of worsening conditions and tailored intervention strategies. Patients gain access to continuous care without frequent in-person visits. The approach improves clinical outcomes, supports preventive care models, and strengthens collaboration between patients, caregivers, and medical professionals.
Customization and Personalization of Device Features
The tremor proof devices market is embracing personalized solutions that cater to varying tremor intensities, underlying conditions, and lifestyle needs. It incorporates modular designs, interchangeable components, and adjustable sensitivity settings. Software-based customization enables updates without hardware replacement, extending device lifecycles. Manufacturers collaborate with clinicians to create patient-specific configurations that optimize functionality. This trend not only enhances treatment effectiveness but also increases patient satisfaction, fostering brand loyalty and positive adoption rates across multiple demographics.
Market Challenges Analysis
High Costs and Limited Accessibility
The tremor proof devices market faces significant challenges due to high production costs, advanced technology integration, and limited manufacturing scale. It often results in premium pricing that restricts accessibility for patients in low- and middle-income regions. Insurance coverage remains inconsistent, with many healthcare systems not fully reimbursing device purchases. Limited distribution networks in rural and emerging markets further slow adoption. Affordability concerns also impact long-term usage, as device maintenance, battery replacements, and upgrades add to the total cost of ownership.
Regulatory Barriers and Lack of Standardization
The tremor proof devices market contends with stringent regulatory requirements and lengthy approval timelines, delaying product launches. It is affected by the absence of standardized performance metrics, making it difficult for healthcare providers and patients to compare products effectively. Variations in regional certification processes increase development complexity for manufacturers targeting global markets. Limited clinical trial data on long-term efficacy also hinders confidence among medical professionals. These factors slow innovation, reduce competitive entry, and limit overall market scalability.
Market Opportunities
Rising Demand in Emerging Economies
The tremor proof devices market holds strong growth potential in emerging economies where healthcare infrastructure is expanding and awareness of assistive technologies is increasing. It benefits from improving diagnostic capabilities, growing middle-class populations, and higher healthcare spending. Governments and NGOs are introducing initiatives to support accessibility for individuals with neurological conditions. Local manufacturing partnerships can reduce costs and improve supply chain efficiency. Increasing adoption in rehabilitation centers and home care settings further broadens the addressable market base in these regions.
Technological Convergence and Product Diversification
The tremor proof devices market is positioned to capitalize on advancements in AI, robotics, and wearable health monitoring technologies. It enables manufacturers to develop multifunctional devices that integrate tremor suppression with broader health tracking features. Expanding customization options for different tremor types can attract diverse patient groups. Opportunities exist in developing subscription-based software updates and remote therapy integrations to extend device lifecycles. Collaborations with healthcare providers and tech companies can accelerate innovation and open new revenue streams.
Market Segmentation Analysis:
By Product Type
The tremor proof devices market is segmented into wearable devices, utensils and tools, assistive technologies, writing aids, and others. Wearable devices lead due to their portability, discreet designs, and integration with smart sensors for real-time tremor control. Utensils and tools address daily living needs, improving eating and self-care activities. Assistive technologies, including motion-stabilizing platforms, serve broader functional requirements. Writing aids remain essential for education and workplace applications, while other solutions target niche needs with specialized functionalities.
- For instance, the Felix™ NeuroAI™ Wristband by Fasikl uses tri-axial accelerometry and AI to monitor tremor patterns and offer personalized electrical stimulation, aiding tremor reduction through nerve stimulation.
By End-User
The tremor proof devices market is categorized into hospitals and clinics, home care settings, educational institutions, and others. Hospitals and clinics dominate due to higher adoption for clinical assessments and rehabilitation programs. Home care settings show strong growth potential, driven by demand for independent living solutions and user-friendly designs. Educational institutions adopt writing aids and assistive tools to support students with tremor disorders. Other end-users include research organizations and therapy centers seeking advanced solutions for specialized applications.
- For instance, the Tremelo™ device, used in clinical environments, mechanically reduces tremor amplitude by up to 85%, supporting patient motor function without requiring electrical power.
Segments:
Based on Product Type:
- Wearable Devices
- Utensils and Tools
- Assistive Technologies
- Writing Aids
- Others
Based on End-User:
- Hospitals and Clinics
- Home Care Settings
- Educational Institutions
- Others
Based on the Geography:
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis
North America
North America holds 38% share of the tremor proof devices market, driven by high awareness of neurological disorders, advanced healthcare infrastructure, and strong adoption of assistive technologies. It benefits from robust reimbursement systems and government support for medical device innovation. The presence of leading manufacturers and research institutions accelerates product development and commercialization. Rising prevalence of Parkinson’s disease and essential tremor fuels sustained demand. Expanding telemedicine networks also facilitate remote device integration, strengthening market reach across urban and rural areas.
Europe
Europe accounts for 27% of the tremor proof devices market, supported by a well-established healthcare system and strong focus on patient quality of life. It experiences high demand for ergonomic and user-friendly designs, particularly in countries with aging populations. EU regulatory frameworks ensure product quality, enhancing patient trust and adoption rates. Manufacturers leverage regional funding programs for R&D in assistive technologies. Cross-border collaborations among healthcare providers and technology firms are fostering faster innovation and deployment.
Asia-Pacific
Asia-Pacific captures 24% share of the tremor proof devices market, fueled by expanding healthcare access, rising middle-class incomes, and growing awareness of neurological care. It benefits from government initiatives promoting assistive technology adoption, especially in Japan, China, and India. Local manufacturing capabilities and cost-effective production models enhance affordability. The increasing prevalence of lifestyle-related neurological conditions supports steady demand. Partnerships between global and regional players are improving product availability and customization for diverse patient needs.
Latin America
Latin America represents 6% of the tremor proof devices market, with growth driven by improving healthcare infrastructure and rising diagnosis rates. It sees gradual adoption in urban centers, where specialist clinics and rehabilitation facilities are more accessible. Economic constraints and limited insurance coverage challenge wider adoption. International aid programs and local NGOs play a role in improving access to assistive devices. Ongoing training for healthcare professionals is fostering better integration of tremor management solutions.
Middle East & Africa
The Middle East & Africa hold 5% share of the tremor proof devices market, with opportunities emerging from growing investment in healthcare modernization. It faces barriers such as low awareness, limited specialist availability, and high device costs. Urban hospitals adopt advanced assistive technologies faster than rural areas. Governments are exploring partnerships with global suppliers to expand access. Rising focus on neurological rehabilitation is expected to gradually increase demand in key markets across the region.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Steadiwear Inc.
- Medtronic plc
- GyroGear Ltd.
- Bioness Inc.
- Lift Labs (Verily Life Sciences LLC)
- Boston Scientific Corporation
- NEOFECT
- Abbott Laboratories
- Penumbra, Inc.
- STMicroelectronics N.V.
Competitive Analysis
The tremor proof devices market is characterized by strong competition among global and regional players focusing on innovation, functionality, and user comfort. It features companies such as Steadiwear Inc., Medtronic plc, GyroGear Ltd., Bioness Inc., Lift Labs (Verily Life Sciences LLC), Boston Scientific Corporation, NEOFECT, Abbott Laboratories, Penumbra, Inc., and STMicroelectronics N.V., each leveraging technology advancements to gain market share. Leading manufacturers invest in AI-driven motion stabilization, lightweight designs, and ergonomic enhancements to meet diverse patient needs. Strategic partnerships with healthcare providers and research institutions accelerate clinical validation and product adoption. Companies are expanding distribution networks, enhancing telemedicine compatibility, and offering customizable solutions to strengthen their competitive positioning. Continuous R&D investment, brand differentiation, and strong after-sales support remain critical for sustaining market leadership and addressing the evolving demands of both clinical and home care segments.
Recent Developments
- In July 2025, Fasikl, a University of Minnesota spin-off, received FDA 510(k) clearance for its Felix™ NeuroAI™ Wristband, an AI-powered, noninvasive wearable designed to reduce essential tremor symptoms through personalized, cloud-based stimulation.
- In July 2025, Neu Health launched its smartphone-based tremor measurement tool for Parkinson’s patients in the U.S. after gaining FDA 510(k) clearance. The tool enables tremor assessment without wearable hardware and is already deployed at Mass General Brigham.
- In April 2025, Steadiwear introduced the Steadi-3, a battery-free wearable glove that stabilizes hand tremors for people with essential tremor, improving daily function and comfort.
- In March 2025, Medtronic secured FDA approval for its BrainSense Adaptive Deep Brain Stimulation (aDBS) system, which delivers real-time therapy adjustments based on brain activity to enhance tremor and Parkinson’s symptom control.
Market Concentration & Characteristics
The tremor proof devices market is moderately concentrated, with a mix of established medical device companies and specialized innovators competing on technology, design, and patient outcomes. It features strong participation from global players such as Steadiwear Inc., Medtronic plc, GyroGear Ltd., Bioness Inc., and Boston Scientific Corporation, alongside niche firms focusing on specific tremor conditions. Competitive differentiation is driven by AI-based motion stabilization, ergonomic product design, and integration with digital health platforms. Market leaders invest heavily in R&D, clinical validation, and strategic partnerships to expand adoption in both clinical and home care environments. Barriers to entry include high development costs, stringent regulatory requirements, and the need for specialized manufacturing capabilities. Pricing pressures are mitigated by the growing demand for premium, personalized solutions that enhance patient independence. Expansion opportunities remain significant in emerging markets, where rising healthcare investments and awareness are gradually increasing product penetration and broadening the competitive landscape.
Report Coverage
The research report offers an in-depth analysis based on Product Type, End-User and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- Rising neurological disorder cases and aging populations will significantly boost demand for tremor proof devices.
- AI-driven motion stabilization features will dominate future product designs, improving accuracy and patient comfort.
- Telemedicine integration will enhance remote monitoring capabilities and enable personalized therapy adjustments for patients.
- Wearable devices will capture greater market share due to portability, discreet design, and daily usability.
- Local manufacturing initiatives will reduce costs, improving affordability and accessibility in developing healthcare markets.
- Regulatory harmonization across regions will speed up global product launches and streamline certification processes.
- Customizable solutions will cater to varying tremor severities, specific conditions, and diverse patient lifestyle requirements.
- Healthcare and technology firm collaborations will drive rapid innovation, clinical validation, and advanced device features.
- Emerging markets will see faster adoption supported by healthcare infrastructure improvements and awareness programs.
- Extended battery life and ergonomic advancements will encourage long-term usage and higher patient satisfaction levels.




