Market Overview
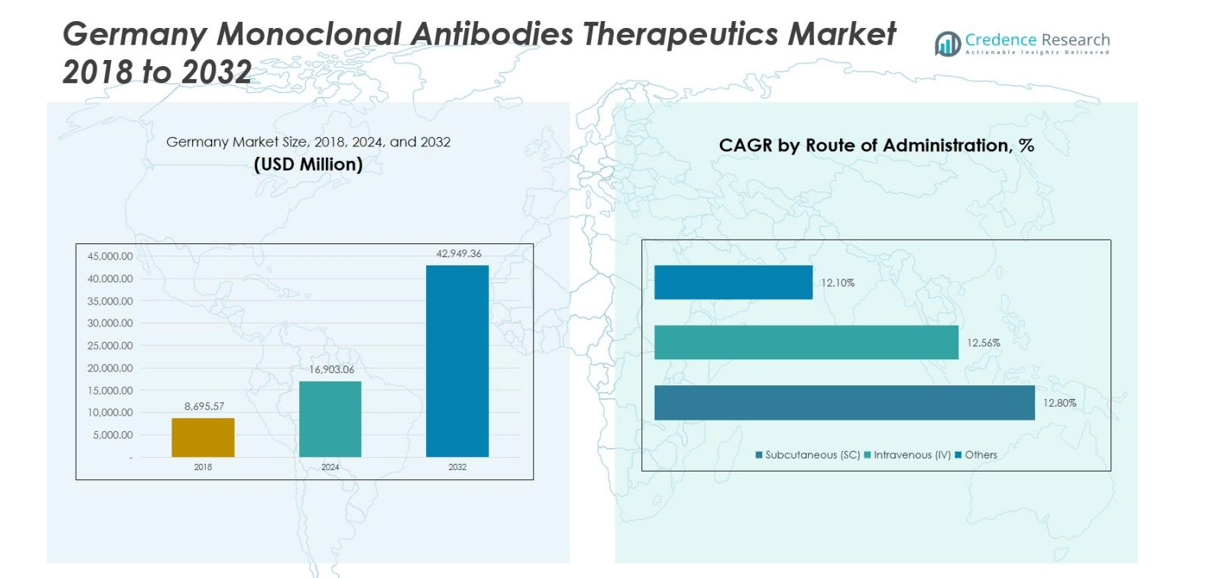
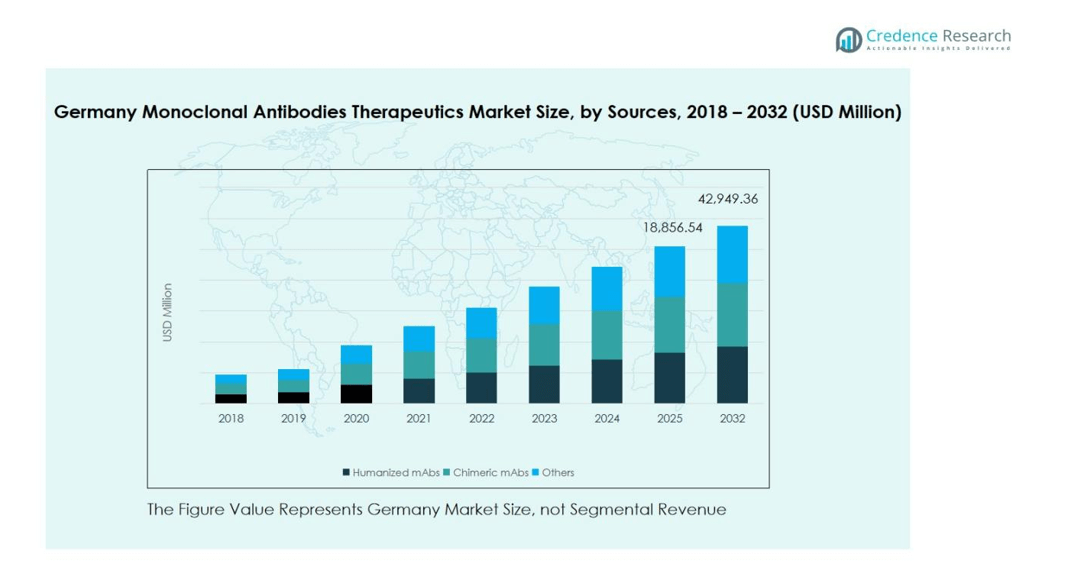
Germany Monoclonal Antibodies Therapeutics Market size was valued at USD 8,695.57 million in 2018, growing to USD 16,903.06 million in 2024, and is anticipated to reach USD 42,949.36 million by 2032, at a CAGR of 12.4% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Germany Monoclonal Antibodies Therapeutics Market Size 2024 |
USD 16,903.06 million |
| Germany Monoclonal Antibodies Therapeutics Market, CAGR |
12.4% |
| Germany Monoclonal Antibodies Therapeutics Market Size 2032 |
USD 42,949.36 million |
The Germany Monoclonal Antibodies Therapeutics Market is dominated by key players such as F. Hoffmann-La Roche Ltd., Sanofi, AbbVie Inc., Bristol Myers Squibb, AstraZeneca, and Novartis AG. These companies maintain strong market positions through extensive R&D pipelines, diverse therapeutic portfolios, and strategic collaborations focused on oncology and autoimmune disorders. Roche leads the competitive landscape with its innovative monoclonal antibody products and robust clinical research presence. Regionally, Southern Germany holds the largest market share at 36%, supported by advanced biotechnology infrastructure, leading pharmaceutical clusters, and a high concentration of manufacturing and research facilities driving innovation and market growth.
Market Insights
- The Germany Monoclonal Antibodies Therapeutics Market was valued at USD 16,903.06 million in 2024 and is projected to reach USD 42,949.36 million by 2032, growing at a CAGR of 12.4%. The oncology segment dominates with 46% market share, while humanized mAbs lead the sources segment at 58%.
- Rising prevalence of cancer, autoimmune, and chronic diseases is driving the demand for targeted monoclonal antibody therapies. Increased patient awareness and healthcare investments are supporting higher adoption rates.
- Key trends include growing adoption of biosimilars and subcutaneous delivery formats, along with a focus on personalized medicine and precision therapies. Expansion of clinical trials is enhancing innovation across the market.
- The market is highly competitive, led by Roche, Sanofi, AbbVie, Bristol Myers Squibb, AstraZeneca, and Amgen, with strategies including R&D investments, collaborations, and product launches strengthening their positions.
- Regionally, Southern Germany leads with 36% share, followed by Western Germany 28%, Northern Germany 21%, and Eastern Germany 15%, driven by infrastructure, research centers, and healthcare facilities.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
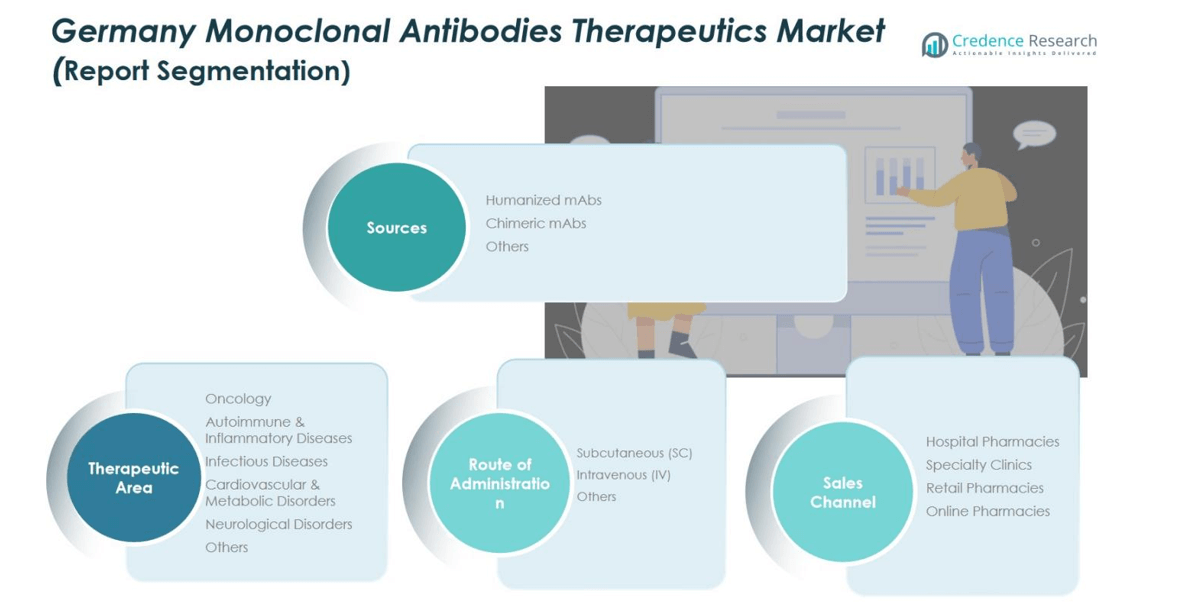
Market Segmentation Analysis:
By Sources:
In the Germany Monoclonal Antibodies Therapeutics Market, humanized monoclonal antibodies (mAbs) dominate the segment, accounting for nearly 58% of the market share in 2024. Their high adoption is attributed to superior efficacy, reduced immunogenicity, and improved patient tolerance compared to chimeric or murine antibodies. The growing prevalence of chronic diseases such as cancer and autoimmune disorders continues to drive demand for advanced antibody formats. Furthermore, ongoing innovation in recombinant DNA technology and rising approvals for humanized antibody-based drugs are strengthening this segment’s leadership position across therapeutic applications.
- For instance, pembrolizumab (Keytruda), a fully humanized mAb, has significantly improved survival rates in triple-negative breast cancer patients, establishing itself as a new standard of care.
By Therapeutic Area:
The oncology segment holds the largest share, representing 46% of the Germany Monoclonal Antibodies Therapeutics Market. This dominance is driven by increasing cancer incidence, government-backed research initiatives, and strong clinical success rates of antibody-based cancer therapies. Rapid advancements in targeted immunotherapies and combination treatment approaches have further accelerated adoption. Autoimmune and inflammatory diseases follow as the second-largest segment, fueled by rising cases of rheumatoid arthritis and psoriasis, along with the expanding use of biologics for long-term disease management.
- For instance, Merck’s pembrolizumab (Keytruda®), widely used in Germany, demonstrated durable long-term survival benefits in certain lung cancer patients across multiple clinical studies, reinforcing its role as a key immune checkpoint inhibitor in oncology.
By Route of Administration:
The intravenous (IV) route leads the Germany Monoclonal Antibodies Therapeutics Market, capturing 63% of the total market share. IV administration remains the preferred method due to its ability to deliver accurate dosages and ensure immediate therapeutic response in hospital settings. The subcutaneous (SC) segment, however, is witnessing steady growth driven by the shift toward self-administration and patient convenience. The increasing development of pre-filled syringes and auto-injectors supports this transition, signaling a gradual movement toward more flexible treatment options outside traditional clinical environments.
Key Growth Drivers
Rising Prevalence of Chronic and Autoimmune Diseases
The increasing incidence of chronic and autoimmune disorders, including cancer, rheumatoid arthritis, and multiple sclerosis, remains a major growth driver of the Germany Monoclonal Antibodies Therapeutics Market. Rising patient awareness, expanding healthcare coverage, and improved diagnostic capabilities have accelerated the adoption of targeted antibody therapies. Monoclonal antibodies are preferred for their precision and lower side effects compared to conventional drugs. Government-backed healthcare programs and a strong hospital infrastructure further reinforce the market’s expansion across multiple therapeutic areas.
- For instance, for rheumatoid arthritis, MOR103, a human monoclonal antibody targeting GM-CSF, demonstrated safety and significant improvements in disease activity in a German clinical trial, indicating strong potential for treating immune-mediated inflammatory diseases.
Advancements in Antibody Engineering and Biomanufacturing
Ongoing technological progress in antibody design and production is significantly strengthening market growth. Innovations such as bispecific antibodies, antibody-drug conjugates, and next-generation recombinant techniques are enhancing drug efficacy and safety. German biotechnology firms are investing in advanced biomanufacturing processes like continuous production and single-use technologies to improve yield and reduce costs. These advancements are enabling faster product development cycles and supporting broader clinical applications in oncology, immunology, and infectious disease management.
- For instance, the continuous clinical advancements in ADCs, where Innovent’s TROP2/PD-L1 bispecific ADC and combination therapies like sacituzumab govitecan plus pembrolizumab demonstrate enhanced targeting and immune activation in oncology.
Growing Focus on Personalized and Precision Medicine
Germany’s shift toward personalized and precision medicine is fueling the demand for monoclonal antibodies tailored to individual patient profiles. The integration of genomics, biomarker testing, and digital health tools is enabling clinicians to identify the most effective therapies. Collaborations between pharmaceutical firms and diagnostic developers are driving the co-development of companion diagnostics, ensuring accurate treatment targeting. This growing emphasis on individualized therapy not only improves patient outcomes but also fosters innovation and enhances therapeutic efficiency across disease categories.
Key Trends & Opportunities
Emergence of Biosimilars and Affordable Therapeutic Options
The increasing presence of biosimilars in Germany’s therapeutic landscape offers substantial opportunities for market expansion. As major monoclonal antibody patents expire, biosimilar manufacturers are introducing affordable alternatives that maintain equivalent safety and efficacy. This shift is improving patient access to life-saving therapies while reducing healthcare expenditure. Supportive government policies and reimbursement incentives for cost-effective biologics are encouraging hospitals and pharmacies to integrate biosimilars into their treatment portfolios, strengthening market competitiveness and long-term sustainability.
- For instance, infliximab biosimilars such as Remsima and Inflectra, approved since 2013, have achieved up to 87% market share in some German regions due to prescription quotas and gain share agreements that incentivize physicians to prescribe cost-effective alternatives while preserving their autonomy.
Expansion of Research Collaborations and Clinical Trials
Germany’s robust research infrastructure and clinical ecosystem are driving partnerships between pharmaceutical companies, academic institutions, and healthcare providers. These collaborations accelerate the development and validation of new monoclonal antibodies across oncology and immunology. The rise in clinical trials not only enhances innovation but also positions Germany as a strategic hub for biologic research and commercialization in Europe. Regulatory support for translational research and funding initiatives is further promoting scientific advancement and product diversification in the market.
- For instance, the Max Delbrück Center for Molecular Medicine (MDC) in Berlin has partnered with Glycotope GmbH to combine Glycotope’s tumor-specific antibodies with MDC’s CAR technology, aiming to enhance immunotherapies for solid tumors.
Key Challenges
High Manufacturing and Development Costs
The complex production process of monoclonal antibodies continues to pose a major challenge for market participants. High costs associated with cell culture systems, purification technologies, and quality control increase overall manufacturing expenses. Smaller biotechnology firms often struggle to scale up due to capital limitations and operational complexities. Furthermore, maintaining batch consistency and meeting strict international quality standards add to production challenges, constraining the availability of affordable therapies and limiting new market entries.
Stringent Regulatory Approval and Reimbursement Barriers
Germany’s rigorous regulatory and reimbursement framework presents a significant obstacle to market growth. Extensive clinical validation requirements and lengthy approval timelines delay product launches. Reimbursement decisions from health authorities demand comprehensive pharmacoeconomic data, particularly for high-cost biologics. These strict conditions slow the adoption of novel monoclonal antibody therapies and increase the burden on manufacturers. As a result, companies must adopt collaborative approaches and strengthen clinical evidence to ensure faster regulatory acceptance and broader patient access.
Regional Analysis
Southern Germany
Southern Germany holds the leading position in the Germany Monoclonal Antibodies Therapeutics Market with a market share of 36%. The region benefits from a strong concentration of biopharmaceutical companies, advanced research institutes, and well-established healthcare infrastructure in cities such as Munich and Stuttgart. High investment in oncology and immunotherapy research, coupled with strong collaborations between academic and clinical institutions, drives regional dominance. Government initiatives supporting precision medicine and the presence of innovation clusters further enhance production capabilities and accelerate clinical adoption of monoclonal antibody-based therapeutics.
Western Germany
Western Germany accounts for 28% of the total market share, supported by its robust pharmaceutical manufacturing base and strong logistics network. The region’s biopharma hubs in North Rhine-Westphalia and Hesse play a central role in clinical trials, product distribution, and large-scale bioprocessing. The growing prevalence of autoimmune and inflammatory disorders is fueling demand for antibody-based drugs. Collaborations between multinational pharmaceutical companies and regional research organizations are driving innovation, while hospitals and specialty clinics continue to expand their use of monoclonal antibodies across oncology and infectious disease treatments.
Northern Germany
Northern Germany captures a market share of 21%, supported by expanding biotechnology research and development activities, particularly in Hamburg and Schleswig-Holstein. The region’s focus on clinical innovation and investment in new biomanufacturing facilities is strengthening its role in the national therapeutic landscape. Increasing partnerships between academic institutions and contract manufacturing organizations are improving supply chain efficiency and accelerating product development. Rising adoption of monoclonal antibodies in hospitals and outpatient settings, supported by efficient healthcare services and patient awareness, continues to drive consistent growth in the region’s therapeutic market.
Eastern Germany
Eastern Germany holds a 15% share of the Germany Monoclonal Antibodies Therapeutics Market. The region is gradually expanding its biotechnology ecosystem through public-private partnerships and government-funded research initiatives. Cities like Leipzig and Dresden are emerging as important centers for clinical research and biologics production. While the market is still developing compared to western regions, increased healthcare investments and improved access to specialty treatments are driving steady growth. Ongoing expansion of pharmaceutical infrastructure and the establishment of innovation parks are expected to enhance the region’s contribution to the national therapeutic landscape.
Market Segmentations:
By Sources
- Humanized mAbs
- Chimeric mAbs
- Others
By Therapeutic Area
- Oncology
- Autoimmune & Inflammatory Diseases
- Infectious Diseases
- Cardiovascular & Metabolic Disorders
- Neurological Disorders
- Others
By Route of Administration
- Subcutaneous (SC)
- Intravenous (IV)
- Others
By Sales Channel
- Hospital Pharmacies
- Specialty Clinics
- Retail Pharmacies
- Online Pharmacies
By Region
- Southern Germany
- Western Germany
- Nortbern Germany
- Eastern Germany
Competitive Landscape
The competitive landscape of the Germany Monoclonal Antibodies Therapeutics Market is characterized by the presence of leading players such as F. Hoffmann-La Roche Ltd, Sanofi, AbbVie Inc., Bristol Myers Squibb Company, AstraZeneca, Amgen Inc., Eli Lilly and Company, Regeneron Pharmaceuticals Inc., and Johnson & Johnson Services, Inc. These companies dominate the market through strong product portfolios, extensive R&D investments, and strategic collaborations. Roche and AbbVie continue to lead in oncology and immunology segments, driven by their extensive monoclonal antibody pipelines and approved therapies. Local biotech firms and research institutions are emerging as critical innovation partners, focusing on biosimilar development and next-generation antibody engineering. Competitive strategies such as mergers, licensing agreements, and expansion of clinical trials in Germany further strengthen market presence. The increasing shift toward personalized medicine and biosimilar production is reshaping competitive dynamics, encouraging both global and domestic players to enhance efficiency and broaden therapeutic applications.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Hoffmann-La Roche Ltd
- Sanofi
- AbbVie Inc.
- Bristol Myers Squibb Company
- CORAT Therapeutics GmbH
- AstraZeneca
- Amgen Inc.
- Eli Lilly and Company
- Regeneron Pharmaceuticals Inc.
- Johnson & Johnson Services, Inc.
- Other Key Players
Recent Developments
- In June 2025, BioNTech and Bristol Myers Squibb entered into a strategic partnership to co-develop and co-commercialize the bispecific antibody BNT327 for multiple solid tumor indications.
- In October 2025, Merck KGaA, Darmstadt, Germany, announced the acquisition of the chromatography business of JSR Life Sciences. This strategic move aims to enhance Merck’s capabilities in bioprocessing and antibody production, aligning with the growing demand for monoclonal antibody therapeutics in Germany.
- On September 30, 2025, VERAXA Biotech and Secarna Pharmaceuticals initiated an alliance to develop antibody-oligonucleotide conjugates targeting autoimmune and inflammatory diseases.
- On March 17, 2025, AstraZeneca announced the acquisition of biotechnology company EsoBiotec for up to $1 billion. This acquisition aims to bolster AstraZeneca’s cell therapy capabilities, enhancing its portfolio in the monoclonal antibody therapeutics sector.
Report Coverage
The research report offers an in-depth analysis based on Sources, Therapeutic Area, Route of Administration, Sales Channel and Region. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market will continue to expand driven by increasing demand for targeted therapies across oncology and autoimmune diseases.
- Ongoing advancements in antibody engineering will enhance treatment efficacy and safety profiles.
- The adoption of biosimilars will increase, improving patient access and reducing overall healthcare costs.
- Personalized medicine will become a key focus, with greater integration of genomic and biomarker-based therapies.
- Germany’s strong clinical research infrastructure will attract more global collaborations and clinical trials.
- Subcutaneous delivery formats will gain traction due to convenience and patient preference for self-administration.
- Biopharmaceutical companies will invest more in sustainable and cost-efficient biomanufacturing technologies.
- Regulatory authorities will emphasize faster approval pathways for innovative antibody therapeutics.
- Public and private sector partnerships will accelerate innovation and market expansion.
- Increasing healthcare expenditure and awareness will support broader adoption of monoclonal antibody treatments nationwide.






