CHAPTER NO. 1: GENESIS OF THE MARKET
1.1 Market Prelude – Introduction & Scope
1.2 The Big Picture – Objectives & Vision
1.3 Strategic Edge – Unique Value Proposition
1.4 Stakeholder Compass – Key Beneficiaries
CHAPTER NO. 2: EXECUTIVE LENS
2.1 Pulse of the Industry – Market Snapshot
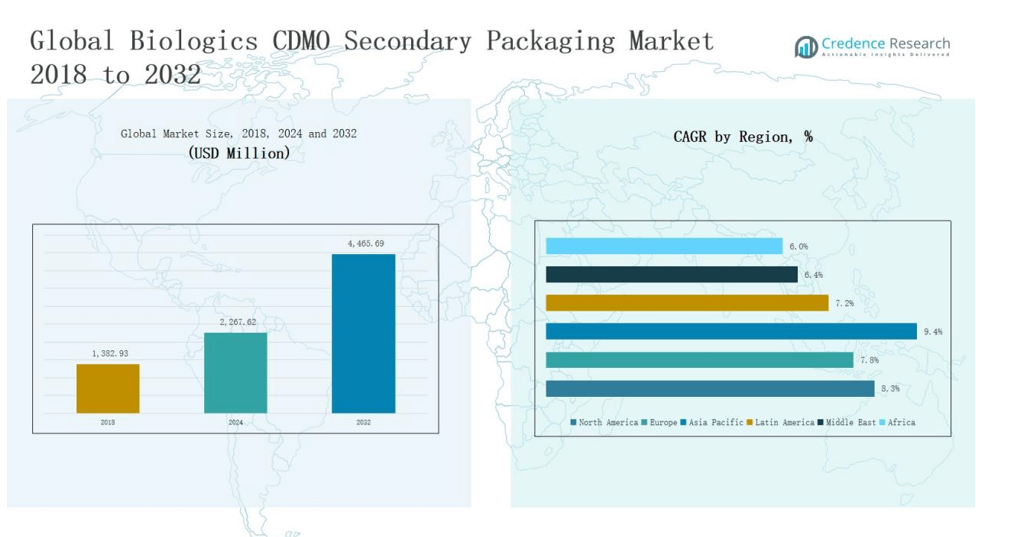
2.2 Growth Arc – Revenue Projections (USD Million)
2.3. Premium Insights – Based on Primary Interviews
CHAPTER NO. 3: BIOLOGICS CDMO SECONDARY PACKAGING FORCES & INDUSTRY PULSE
3.1 Foundations of Change – Market Overview
3.2 Catalysts of Expansion – Key Market Drivers
3.2.1 Momentum Boosters – Growth Triggers
3.2.2 Innovation Fuel – Disruptive Technologies
3.3 Headwinds & Crosswinds – Market Restraints
3.3.1 Regulatory Tides – Compliance Challenges
3.3.2 Economic Frictions – Inflationary Pressures
3.4 Untapped Horizons – Growth Potential & Opportunities
3.5 Strategic Navigation – Industry Frameworks
3.5.1 Market Equilibrium – Porter’s Five Forces
3.5.2 Ecosystem Dynamics – Value Chain Analysis
3.5.3 Macro Forces – PESTEL Breakdown
3.6 Price Trend Analysis
3.6.1 Regional Price Trend
3.6.2 Price Trend by Secondary Packaging Type
CHAPTER NO. 4: KEY INVESTMENT EPICENTER
4.1 Regional Goldmines – High-Growth Geographies
4.2 Product Frontiers – Lucrative Product Categories
4.3 Application Sweet Spots – Emerging Demand Segments
CHAPTER NO. 5: REVENUE TRAJECTORY & WEALTH MAPPING
5.1 Momentum Metrics – Forecast & Growth Curves
5.2 Regional Revenue Footprint – Market Share Insights
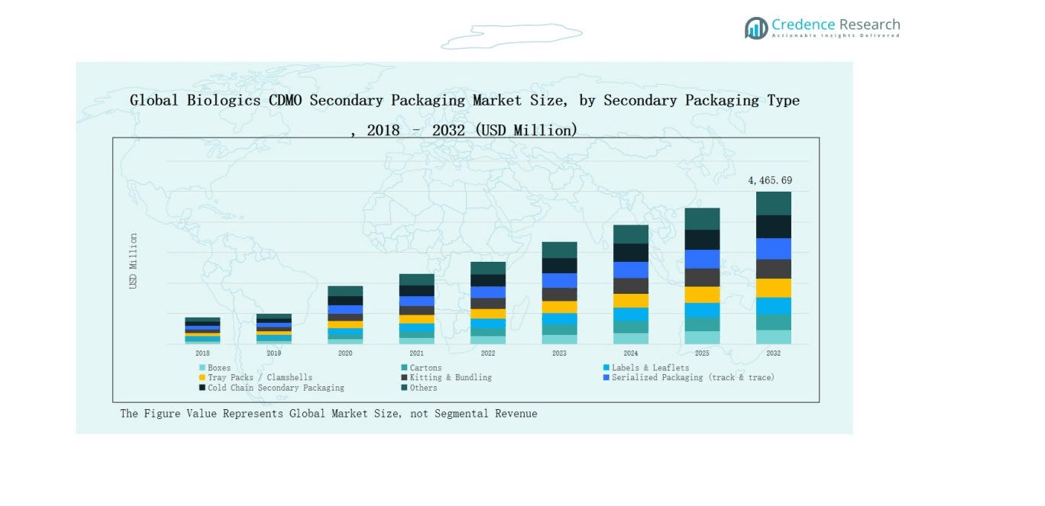
5.3 Segmental Wealth Flow – Secondary Packaging Type & Secondary Packaging by Compatible Primary Package Type Revenue
CHAPTER NO. 6 : TRADE & COMMERCE ANALYSIS
6.1. Import Analysis by Region
6.1.1. Global Biologics CDMO Secondary Packaging Market Import Revenue By Region
6.2. Export Analysis by Region
6.2.1. Global Biologics CDMO Secondary Packaging Market Export Revenue By Region
CHAPTER NO. 7: COMPETITION ANALYSIS
7.1. Company Market Share Analysis
7.1.1. Global Biologics CDMO Secondary Packaging Market: Company Market Share
7.2. Global Biologics CDMO Secondary Packaging Market Company Revenue Market Share
7.3. Strategic Developments
7.3.1. Acquisitions & Mergers
7.3.2. New Product Type Launch
7.3.3. Regional Expansion
7.4. Competitive Dashboard
7.5. Company Assessment Metrics, 2024
CHAPTER NO. 8: BIOLOGICS CDMO SECONDARY PACKAGING – BY SECONDARY PACKAGING TYPE SEGMENT ANALYSIS
8.1. Biologics CDMO Secondary Packaging Market Overview by Secondary Packaging Type Segment
8.1.1. Biologics CDMO Secondary Packaging Market Revenue Share By Secondary Packaging Type
8.2. Boxes
8.3. Cartons
8.4. Labels & Leaflets
8.5. Tray Packs / Clamshells
8.6. Kitting & Bundling
8.7. Serialized Packaging (track & trace)
8.8. Cold Chain Secondary Packaging
8.9. Others
CHAPTER NO. 9: BIOLOGICS CDMO SECONDARY PACKAGING – BY SECONDARY PACKAGING BY COMPATIBLE PRIMARY PACKAGE TYPE SEGMENT ANALYSIS
9.1. Biologics CDMO Secondary Packaging Market Overview by Secondary Packaging by Compatible Primary Package Type Segment
9.1.1. Biologics CDMO Secondary Packaging Market Revenue Share By Secondary Packaging by Compatible Primary Package Type
9.2. Ampoules
9.3. Blister packs
9.4. Cartridges
9.5. Vials
9.6. Bottles
9.7. Prefilled Syringes
9.8. Others
CHAPTER NO. 10: BIOLOGICS CDMO SECONDARY PACKAGING – REGIONAL ANALYSIS
10.1. Biologics CDMO Secondary Packaging Market Overview by Region Segment
10.1.1. Global Biologics CDMO Secondary Packaging Market Revenue Share By Region
10.1.2. Region
10.1.3. Secondary Packaging Type
10.1.4. Global Biologics CDMO Secondary Packaging Market Revenue By Secondary Packaging Type
10.1.5. Secondary Packaging by Compatible Primary Package Type
10.1.6. Global Biologics CDMO Secondary Packaging Market Revenue By Secondary Packaging by Compatible Primary Package Type
CHAPTER NO. 11: NORTH AMERICA BIOLOGICS CDMO SECONDARY PACKAGING – COUNTRY ANALYSIS
11.1. North America Biologics CDMO Secondary Packaging Market Overview by Country Segment
11.1.1. North America Biologics CDMO Secondary Packaging Market Revenue Share By Region
11.2. North America
11.2.1. North America Biologics CDMO Secondary Packaging Market Revenue By Country
11.2.2. Secondary Packaging Type
11.2.3. North America Biologics CDMO Secondary Packaging Market Revenue By Secondary Packaging Type
11.2.4. Secondary Packaging by Compatible Primary Package Type
11.2.5. North America Biologics CDMO Secondary Packaging Market Revenue By Secondary Packaging by Compatible Primary Package Type
11.3. U.S.
11.4. Canada
11.5. Mexico
CHAPTER NO. 12: EUROPE BIOLOGICS CDMO SECONDARY PACKAGING – COUNTRY ANALYSIS
12.1. Europe Biologics CDMO Secondary Packaging Market Overview by Country Segment
12.1.1. Europe Biologics CDMO Secondary Packaging Market Revenue Share By Region
12.2. Europe
12.2.1. Europe Biologics CDMO Secondary Packaging Market Revenue By Country
12.2.2. Secondary Packaging Type
12.2.3. Europe Biologics CDMO Secondary Packaging Market Revenue By Secondary Packaging Type
12.2.4. Secondary Packaging by Compatible Primary Package Type
12.2.5. Europe Biologics CDMO Secondary Packaging Market Revenue By Secondary Packaging by Compatible Primary Package Type
12.3. UK
12.4. France
12.5. Germany
12.6. Italy
12.7. Spain
12.8. Russia
12.9. Rest of Europe
CHAPTER NO. 13: ASIA PACIFIC BIOLOGICS CDMO SECONDARY PACKAGING – COUNTRY ANALYSIS
13.1. Asia Pacific Biologics CDMO Secondary Packaging Market Overview by Country Segment
13.1.1. Asia Pacific Biologics CDMO Secondary Packaging Market Revenue Share By Region
13.2. Asia Pacific
13.2.1. Asia Pacific Biologics CDMO Secondary Packaging Market Revenue By Country
13.2.2. Secondary Packaging Type
13.2.3. Asia Pacific Biologics CDMO Secondary Packaging Market Revenue By Secondary Packaging Type
13.2.4. Secondary Packaging by Compatible Primary Package Type
13.2.5. Asia Pacific Biologics CDMO Secondary Packaging Market Revenue By Secondary Packaging by Compatible Primary Package Type
13.3. China
13.4. Japan
13.5. South Korea
13.6. India
13.7. Australia
13.8. Southeast Asia
13.9. Rest of Asia Pacific
CHAPTER NO. 14: LATIN AMERICA BIOLOGICS CDMO SECONDARY PACKAGING – COUNTRY ANALYSIS
14.1. Latin America Biologics CDMO Secondary Packaging Market Overview by Country Segment
14.1.1. Latin America Biologics CDMO Secondary Packaging Market Revenue Share By Region
14.2. Latin America
14.2.1. Latin America Biologics CDMO Secondary Packaging Market Revenue By Country
14.2.2. Secondary Packaging Type
14.2.3. Latin America Biologics CDMO Secondary Packaging Market Revenue By Secondary Packaging Type
14.2.4. Secondary Packaging by Compatible Primary Package Type
14.2.5. Latin America Biologics CDMO Secondary Packaging Market Revenue By Secondary Packaging by Compatible Primary Package Type
14.3. Brazil
14.4. Argentina
14.5. Rest of Latin America
CHAPTER NO. 15: MIDDLE EAST BIOLOGICS CDMO SECONDARY PACKAGING – COUNTRY ANALYSIS
15.1. Middle East Biologics CDMO Secondary Packaging Market Overview by Country Segment
15.1.1. Middle East Biologics CDMO Secondary Packaging Market Revenue Share By Region
15.2. Middle East
15.2.1. Middle East Biologics CDMO Secondary Packaging Market Revenue By Country
15.2.2. Secondary Packaging Type
15.2.3. Middle East Biologics CDMO Secondary Packaging Market Revenue By Secondary Packaging Type
15.2.4. Secondary Packaging by Compatible Primary Package Type
15.2.5. Middle East Biologics CDMO Secondary Packaging Market Revenue By Secondary Packaging by Compatible Primary Package Type
15.3. GCC Countries
15.4. Israel
15.5. Turkey
15.6. Rest of Middle East
CHAPTER NO. 16: AFRICA BIOLOGICS CDMO SECONDARY PACKAGING – COUNTRY ANALYSIS
16.1. Africa Biologics CDMO Secondary Packaging Market Overview by Country Segment
16.1.1. Africa Biologics CDMO Secondary Packaging Market Revenue Share By Region
16.2. Africa
16.2.1. Africa Biologics CDMO Secondary Packaging Market Revenue By Country
16.2.2. Secondary Packaging Type
16.2.3. Africa Biologics CDMO Secondary Packaging Market Revenue By Secondary Packaging Type
16.2.4. Secondary Packaging by Compatible Primary Package Type
16.2.5. Africa Biologics CDMO Secondary Packaging Market Revenue By Secondary Packaging by Compatible Primary Package Type
16.3. South Africa
16.4. Egypt
16.5. Rest of Africa
CHAPTER NO. 17: COMPANY PROFILES
17.1. WuXi Biologics (Cayman) Inc.
17.1.1. Company Overview
17.1.2. Secondary Packaging Type Portfolio
17.1.3. Financial Overview
17.1.4. Recent Developments
17.1.5. Growth Strategy
17.1.6. SWOT Analysis
17.2. FUJIFILM Diosynth Biotechnologies U.S.A. Inc.
17.3. Samsung Biologics Co. Ltd.
17.4. Thermo Fisher Scientific Inc.
17.5. Catalent, Inc.
17.6. Lonza Group
17.7. PCI Pharma Services