Market Overview
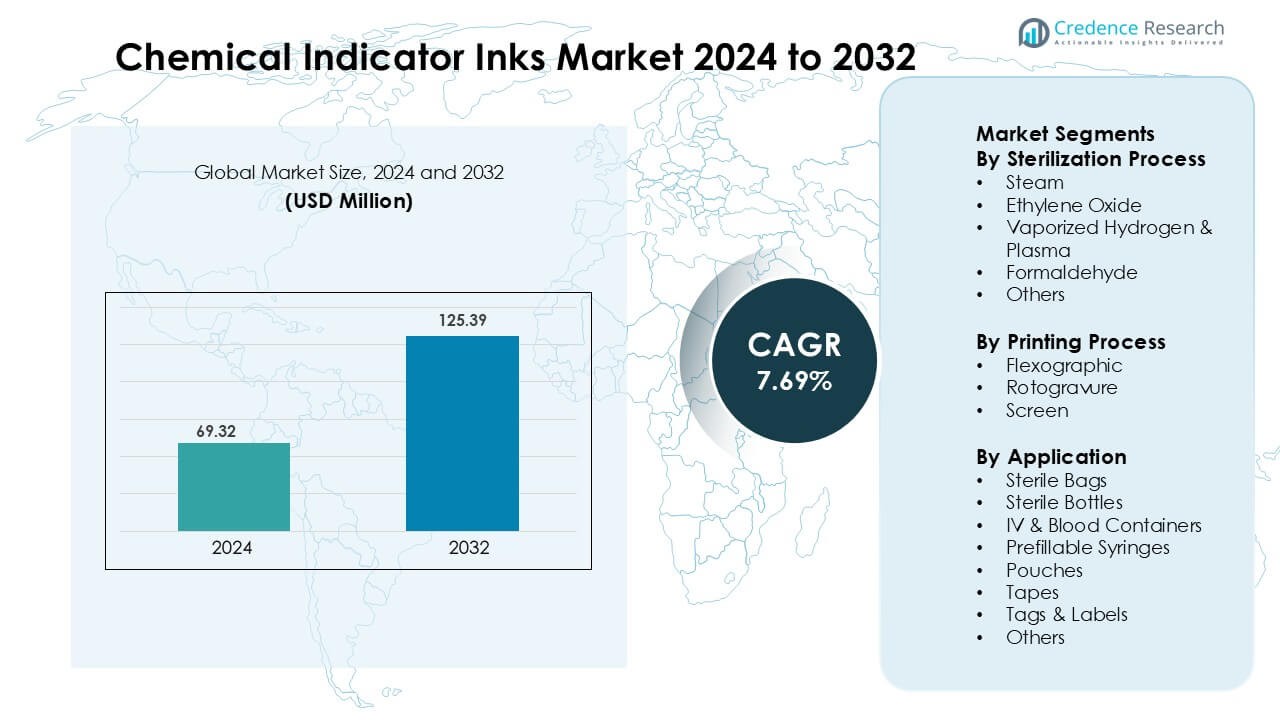
Chemical Indicator Inks Market was valued at USD 69.32 million in 2024 and is anticipated to reach USD 125.39 million by 2032, growing at a CAGR of 7.69% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Chemical Indicator Inks Market Size 2024 |
USD 69.32 Million |
| Chemical Indicator Inks Market, CAGR |
7.69% |
| Chemical Indicator Inks Market Size 2032 |
USD 125.39 Million |
The Chemical Indicator Inks Market is shaped by major suppliers such as 3M, North American Science Associates Inc. (NAMSA), Propper Manufacturing Company, SteriTec Products, GKE GmbH, Terragene SA, NiGK Corporation, Tempil (LA-Co Industries), Riken Chemical Co Ltd., and ETIGAM BV. These companies provide certified indicator inks for steam, ethylene oxide, plasma, and low-temperature sterilization, offering strong color contrast and high chemical stability for medical tapes, pouches, and labels. North America remains the leading regional market, holding 35% of global revenue, driven by rigorous infection control standards, strong hospital sterilization practices, and advanced medical packaging infrastructure.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The Chemical Indicator Inks Market was valued at USD 69.32 Million in 2024 and is projected to reach USD 125.39 Million by 2032, registering a CAGR of 7.69%.
- Strong infection control rules in hospitals and medical device manufacturing push demand for indicator inks used on tapes, pouches, and labels. Steam sterilization remains the largest process segment with 40% share, supported by high surgical volumes.
- Growing adoption of plasma and vaporized hydrogen peroxide sterilization creates opportunities for multi-process inks designed for sensitive instruments and medical polymers.
- Competition centers on certified ink systems, sharp color contrast, and solvent-free formulations. Leading players include 3M, NAMSA, Propper Manufacturing, Terragene, GKE GmbH, SteriTec, and NiGK Corporation.
- North America dominates with 35% market share, followed by Europe at 30%, supported by strict hygiene standards and advanced printing infrastructure. Asia-Pacific grows fastest due to expanding hospitals and medical packaging production.
Market Segmentation Analysis:
By Sterilization Process
Steam sterilization holds the dominant share of the chemical indicator inks market, accounting for nearly 40% of revenue. Healthcare facilities and medical device makers prefer steam-based processes because they deliver reliable microbial elimination, low operating cost, and compatibility with common packaging materials. Indicator inks used in steam sterilization provide clear visual color change, fast response time, and stability under high temperature and pressure. Growing surgical procedures, rising use of sterilizable medical instruments, and strict sterilization audits in hospitals push demand for steam-compatible indicator inks. The segment benefits from continuous upgrades in water-based and non-toxic formulations.
- For instance, 3M’s Comply SteriGage Class 5 steam indicator responds to critical parameters including a minimum of 121°C for 15 minutes, showing a clear color transition when temperature and saturated steam conditions are met.
By Printing Process
Flexographic printing represents the largest share, close to 45%, due to high production speed and low per-unit cost. Medical packaging converters use flexographic systems to print clear, durable sterilization indicators on tapes, pouches, bags, and labels. The process supports water-based and UV-curable indicator inks, ensuring sharp color change after exposure to steam, ethylene oxide, or plasma. Its ability to handle a wide range of substrates such as Tyvek, medical-grade paper, and films drives higher adoption. Demand rises as packaging suppliers expand automated, high-volume production for hospitals and contract sterilization centers.
- For instance, ETIGAM BV guarantees a shelf life of 24 months for its sterilization process indicators (unprocessed) from the date of production, provided they are stored under proper conditions.
By Application
Tapes lead the application segment with nearly 30% market share. Sterilization indicator tapes are widely used in hospitals, clinics, and laboratories to seal packs and visually confirm exposure to a sterilization cycle. Chemical indicator inks on these tapes change color when exposed to temperature or gas levels, helping staff verify process completion. High usage frequency, low cost, and compatibility with steam and ethylene oxide sterilization support growth. Rising healthcare facility expansions and strict infection control policies strengthen demand for indicator tapes in both developed and emerging markets.
Key Growth Drivers
Rising Infection Control Standards and Regulatory Compliance
Hospitals and medical device manufacturers face strict rules on sterilization. Governments and healthcare regulators mandate validated sterilization cycles for surgical tools, implantable devices, and laboratory equipment. Chemical indicator inks provide quick visual proof that sterilization conditions were achieved, which reduces contamination risk and supports audit compliance. Growing surgical volumes, frequent use of reusable instruments, and risk of hospital-acquired infections push demand for reliable indicator inks. Many healthcare facilities also shift to single-use sterile packaging, creating higher printing requirements for color-changing labels, tapes, and pouches. As regulatory policies strengthen in North America, Europe, and Asia, manufacturers invest in inks that show sharper color contrast, faster response time, and improved chemical stability. This consistent adoption reinforces long-term market growth.
- For instance, Propper Manufacturing’s Class 4 steam indicator ink is validated to respond at 121°C for 15 minutes and 134°C for 3 minutes, meeting ISO 11140-1 performance criteria.
Expansion of Sterile Medical Packaging and Life Science Logistics
Sterile packaging has expanded across medical devices, diagnostics, pharmaceuticals, and biotechnology supplies. Contract sterilization service providers and packaging converters print indicator inks on pouches, sterile bags, and blister packs to ensure traceability from factory to hospital. The sharp rise in prefillable syringes, blood bags, IV containers, and implant packaging drives repetitive printing demand. Cold-chain logistics and life science transportation add further need for visual sterilization confirmation on shipped kits and consumables. Increasing outsourcing of medical device sterilization in developing regions deepens commercial usage of steam- and EO-compatible indicator inks. Packaging suppliers adopt digital and flexographic printing to handle high volumes without compromising print clarity, helping the market expand into diverse medical and lab environments.
- For instance, Terragene SA does indeed supply a wide range of both chemical and biological indicators for various sterilization processes, and the BT220 biological indicator is a specific product within their catalog.
Shift Toward Eco-Friendly and Non-Toxic Ink Formulations
Healthcare sustainability programs push manufacturers to eliminate solvent-heavy sterilization inks. Producers now provide water-based, non-toxic, and heavy-metal-free formulations with strong heat and gas resistance. These inks show rapid, stable color change while reducing harmful emissions during printing. Healthcare packaging companies and hospitals increasingly prefer safer chemicals for patient and worker protection. Stringent disposal regulations also encourage low-VOC and non-hazardous indicator inks. The shift supports wider adoption across sensitive applications such as pediatric devices and pharmaceutical packaging. Companies that meet both environmental and regulatory standards gain faster approvals and better market presence. As sustainability becomes part of procurement policies, eco-friendly indicator inks gain long-term growth momentum.
Key Trends & Opportunities
Growing Adoption of Advanced Sterilization Technologies
Healthcare providers adopt plasma, vaporized hydrogen peroxide, and low-temperature gas sterilization to protect heat-sensitive instruments. Chemical indicator inks must deliver accurate and fast color shifts under these alternative sterilization environments. The expansion of robotic surgery tools, plastic-based devices, and electronic medical instruments opens new opportunities for specialized indicator inks. Manufacturers develop formulations tailored for specific sterilization parameters such as humidity, temperature, and gas concentration. As hospitals modernize sterilization centers, demand rises for multi-process indicator inks that work with steam, EO, plasma, and formaldehyde in a single product line. Product portfolios with wider compatibility improve purchasing efficiency and increase market penetration.
- For instance, Terragene’s product lines, such as the Chemdye® CD42 Process Indicators and CDS47V indicators, are designed for monitoring Plasma or Vaporized Hydrogen Peroxide processes.
Growing Use of Automated High-Speed Printing in Medical Packaging
Medical packaging suppliers deploy automated flexographic, rotogravure, and digital lines to meet rising order volumes. Indicator inks must support high-speed printing while maintaining sharp color contrast and print durability. The trend opens opportunities for UV-curable and quick-drying formulations that reduce downtime and waste. Precision printing becomes important as more companies add sterilization-ready labels, barcodes, and traceability elements. The market benefits from greater investment in smart packaging and track-and-trace systems, linking sterilization indicators with digital records. Faster production cycles and efficiency-focused packaging workflows create opportunities for ink manufacturers offering reliable, high-volume solutions.
- For instance, ETIGAM BV’s steam indicators are designed for use in autoclaves operating at specific conditions, such as 134°C for 3 minutes (or 121°C/1 bar for a different time), and undergo a color change from pink to dark brown.
Key Challenges
Performance Limitations Under Extreme Sterilization Conditions
Some indicator inks struggle with performance when exposed to highly variable sterilization conditions, including high humidity, low pressure, and mixed gas environments. If inks show weak or ambiguous color change, healthcare workers may misinterpret sterilization completion, leading to safety risks. Developing robust inks that provide clear visual shifts across multiple sterilization processes increases research cost and formulation complexity. Manufacturers must balance chemical stability, printability, drying time, and regulatory compliance. These challenges slow product approvals and raise production expenses, making innovation difficult for small and mid-sized suppliers.
Volatile Raw Material Prices and Supply Chain Pressure
Chemical indicator ink production depends on specialty pigments, solvents, binders, and UV-active compounds. Price volatility in these inputs affects manufacturing cost and profit margins. Disruptions in global supply chains, particularly for medical-grade pigments and imported chemicals, create delivery delays and inventory shortages. Healthcare packaging customers expect guaranteed sterilization safety and cannot tolerate print defects or inconsistent color shifts. To meet demand, manufacturers must maintain strict quality control, carry higher raw material stock, and invest in alternate sourcing. These operational pressures increase market complexity and limit cost flexibility, especially for emerging suppliers.
Regional Analysis
North America
North America holds the leading share of the Chemical Indicator Inks Market at nearly 35%. High infection control standards, strong surgical volumes, and widespread use of sterilizable medical devices drive demand. Hospitals and contract sterilization centers rely on indicator inks for tapes, pouches, labels, and prefillable syringes. The region benefits from advanced packaging converters with high-speed flexographic printing lines supporting steam and EO sterilization. Regulatory oversight from FDA and CDC encourages adoption of reliable color-changing inks with proven performance. Manufacturers invest in water-based, non-toxic formulations, positioning North America as a hub for innovation.
Europe
Europe accounts for roughly 30% of the market. Strict medical packaging regulations, strong hospital hygiene protocols, and active sterilization audits drive steady usage. Major healthcare systems in Germany, the U.K., and France rely on chemical indicator inks for reusable surgical tools and sterile packaging. Growth in plasma and vaporized hydrogen peroxide sterilization fuels demand for compatible inks. Sustainability policies accelerate the shift toward solvent-free and heavy-metal-free formulations. Advanced printing infrastructure and expanding contract sterilization services make Europe a key revenue contributor.
Asia-Pacific
Asia-Pacific captures close to 25% market share and remains the fastest-growing region. Expanding healthcare infrastructure, rising hospital admissions, and increasing surgical procedures boost consumption of indicator tapes, pouches, and labels. China, India, and Southeast Asia invest in sterile packaging for medical devices, diagnostics, and pharmaceuticals. Local packaging converters adopt automated flexographic printing to meet export standards. Growing pharmaceutical production and medical tourism also support market expansion. As regulatory enforcement strengthens, demand for certified, high-contrast indicator inks continues to accelerate.
Latin America
Latin America holds around 5% market share. The market gains support from improving hospital sterilization standards and higher usage of sterile pouches and medical tapes. Brazil and Mexico drive most consumption due to larger healthcare networks and growing private hospital investments. Adoption is slower compared to developed regions, but rising infection control awareness and new surgical facilities create future growth potential. Import-dependent ink supplies and cost sensitivity remain key barriers.
Middle East & Africa
The Middle East & Africa represents nearly 5% of the market. Growth is driven by expanding hospital infrastructure, rising medical tourism, and increasing purchase of sterilizable medical devices. Gulf countries invest in advanced sterilization centers and high-quality medical packaging. However, limited local manufacturing and reliance on imports slow wider adoption. International suppliers target the region with steam and EO compatible indicator inks, helping healthcare facilities meet safety certifications and audit requirements.
Market Segmentations
By Sterilization Process
- Steam
- Ethylene Oxide
- Vaporized Hydrogen & Plasma
- Formaldehyde
- Others
By Printing Process
- Flexographic
- Rotogravure
- Screen
By Application
- Sterile Bags
- Sterile Bottles
- IV & Blood Containers
- Prefillable Syringes
- Pouches
- Tapes
- Tags & Labels
- Others
By Geography
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Competitive Landscape
The Chemical Indicator Inks Market features a mix of global medical suppliers, specialty ink formulators, and sterilization technology companies. Key players such as 3M, NAMSA, NiGK Corporation, Terragene, SteriTec Products, and Propper Manufacturing focus on certified indicator systems for steam, ethylene oxide, plasma, and formaldehyde sterilization. Many companies expand portfolios with multi-parameter inks that provide faster and sharper color transitions. High-speed flexographic and digital print compatibility has become a priority as converters seek efficient production for tapes, pouches, and labels. Sustainability drives investment in water-based and solvent-free formulations, while regulatory compliance pushes labs to validate color accuracy and chemical stability. Partnerships with medical device manufacturers and sterilization service providers help suppliers gain long-term contracts. Emerging regional companies in Asia and Latin America compete through cost-effective solutions, though global brands maintain advantage through product testing, traceability systems, and extensive distribution networks.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- GKE GmbH
- Propper Manufacturing Company, Inc.
- Terragene SA
- NiGK Corporation
- Tempil (LA-Co Industries)
- ETIGAM BV
- 3M
- North American Science Associates Inc. (NAMSA)
- SteriTec Products Inc.
- Riken Chemical Co Ltd.
Recent Developments
- In May 2025, Terragene launched Chemink IK17F/IK17S EO indicator inks. The Type 1 inks shift from blue to green under validated EO conditions. Pages highlight flexography and serigraphy printing options.
- In September 2024, Proper gains FDA clearance for EO Chex ethylene-oxide indicator tape. The tape pink chemical indicator ink turns orange-brown after EO exposure. Sizes include ¾-inch and 1-inch rolls.
Report Coverage
The research report offers an in-depth analysis based on Sterilization Process, Printing Process, Application and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- Demand will increase as hospitals tighten sterilization audits and surgical volumes rise.
- Water-based and solvent-free formulations will gain wider adoption due to sustainability goals.
- Multi-process indicator inks compatible with steam, EO, plasma, and VHP will see faster uptake.
- High-speed flexographic and digital printing lines will drive need for rapid-drying, high-contrast inks.
- Growth in sterile packaging for prefillable syringes, IV containers, and blood bags will support market expansion.
- Asia-Pacific will remain the fastest-growing region as healthcare infrastructure improves.
- New formulations will focus on sharper color change and improved chemical stability under extreme conditions.
- Partnerships between ink makers and contract sterilization facilities will strengthen long-term supply chains.
- Manufacturers will invest in non-toxic raw materials to meet stricter environmental and disposal regulations.
- Small and regional suppliers will enter the market with cost-efficient solutions, increasing price competition.




