Market Overview
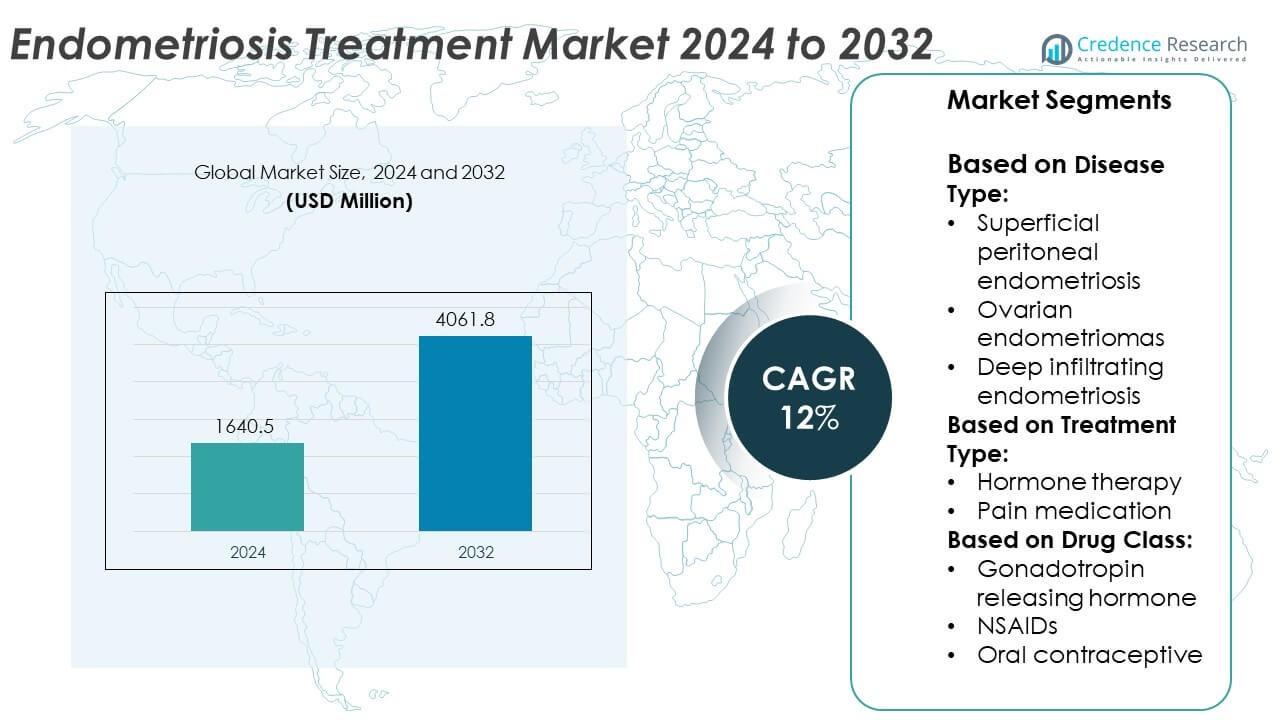
The Endometriosis Treatment Market size was valued at USD 1640.5 million in 2024 and is anticipated to reach USD 4061.8 million by 2032, growing at a CAGR of 12% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2024 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Endometriosis Treatment Market Size 2024 |
USD 1640.5 Million |
| Endometriosis Treatment Market, CAGR |
12% |
| Endometriosis Treatment Market Size 2032 |
USD 4061.8 Million |
The Endometriosis Treatment market grows due to rising demand for advanced, safe, and reliable components in medical devices used for diagnosis and treatment. Increasing prevalence of endometriosis and expanding healthcare infrastructure drive adoption across hospitals and specialized clinics. It benefits from technological advancements such as compact designs, IoT-enabled monitoring, and improved energy efficiency, enhancing operational precision. Trends include greater integration with automated treatment systems, compliance with stringent regulatory standards, and a shift toward eco-friendly manufacturing practices.
North America leads the Endometriosis Treatment market, supported by advanced healthcare infrastructure and strong adoption of innovative medical technologies. Europe follows with high regulatory standards and widespread use of specialized treatment equipment, while Asia-Pacific shows rapid growth driven by expanding healthcare access and rising awareness of women’s health. Latin America and the Middle East & Africa demonstrate steady development through modernization and private sector investment. Key players shaping the market include Astellas Pharma, Inc., AbbVie, Inc., and Pfizer, Inc.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The Endometriosis Treatment market was valued at USD 1,640.5 million in 2024 and is projected to reach USD 4,061.8 million by 2032, registering a CAGR of 12% during the forecast period.
- Growing prevalence of endometriosis and the rising demand for safe, reliable components in advanced medical devices drive market expansion across hospitals, clinics, and specialized treatment centers.
- Technological advancements, including compact designs, IoT-enabled monitoring, and energy-efficient features, are shaping product development and improving operational precision in treatment procedures.
- The market features strong competition with key players such as Astellas Pharma, Inc., AbbVie, Inc., Pfizer, Inc., and Bayer AG focusing on innovation, strategic alliances, and expanding product portfolios to strengthen their positions.
- High production costs, complex integration with existing systems, and stringent regulatory compliance requirements restrain market growth, particularly in cost-sensitive regions and smaller healthcare facilities.
- North America leads due to advanced healthcare infrastructure and rapid adoption of innovative solutions, followed by Europe with high-quality standards and clinical readiness, while Asia-Pacific experiences rapid growth supported by healthcare modernization and increasing awareness.
- Manufacturers are prioritizing regional partnerships, compliance with evolving safety standards, and sustainable manufacturing practices to capture emerging opportunities in both developed and developing markets.
Market Drivers
Rising Prevalence of Endometriosis and Increasing Demand for Advanced Treatment Solutions
The Endometriosis Treatment market benefits from the growing prevalence of endometriosis worldwide. Increasing awareness among healthcare providers and patients fuels demand for effective and reliable treatment options. It supports the operation of medical devices critical in endometriosis management, ensuring safety and efficiency during procedures. Innovations in disconnect switch technology enhance compatibility with advanced treatment equipment, promoting wider adoption. Hospitals and specialized clinics invest in modern solutions to improve patient outcomes. The market growth aligns with ongoing efforts to minimize procedural risks and improve device control during treatments.
- For instance, endometriosis is estimated to affect 10% of reproductive age women, which is approximately 42 million women in India. The number is rising, which increases the demand for effective treatments.
Technological Advancements Driving Enhanced Safety and Efficiency
Technological progress in disconnect switches plays a significant role in the market’s expansion. Improved designs focus on compactness, durability, and ease of integration with endometriosis treatment devices. It enables seamless power management and quick disconnection in emergencies, which is crucial during sensitive medical procedures. Manufacturers incorporate advanced materials and features that meet stringent safety standards, enhancing device reliability. These innovations increase the demand for disconnect switches tailored specifically for the healthcare sector. The adoption of smart and automated disconnect switch systems further strengthens market momentum.
- For instance, Astellas completed an early feasibility study from concept to IDE approval within 4 years, demonstrating its agility in advancing bioelectronic technologies.
Stringent Regulatory Frameworks and Compliance Requirements
Strict regulatory standards governing medical device safety directly impact the Endometriosis Treatment market. Regulatory bodies enforce rigorous testing and certification processes, ensuring that disconnect switches meet high safety and performance benchmarks. It compels manufacturers to innovate and maintain quality assurance throughout the production cycle. Compliance with global and regional regulations facilitates market access and boosts consumer confidence. Healthcare providers prioritize certified and reliable disconnect switch components to avoid operational risks. The emphasis on regulatory adherence drives continuous improvements in product design and functionality.
Expanding Healthcare Infrastructure and Increasing Investments
Growth in healthcare infrastructure worldwide supports the demand for specialized medical equipment, including disconnect switches used in endometriosis treatments. Investments in hospital modernization and expansion create opportunities for advanced device integration. It allows healthcare facilities to upgrade existing systems, incorporating disconnect switches that enhance operational safety. The rise in government and private sector funding for women’s health initiatives further fuels market growth. Developing regions show increasing adoption due to improved healthcare access and awareness. These factors collectively strengthen the market’s trajectory over the forecast period.
Market Trends
Integration of Smart Technologies Enhances Operational Precision
The Endometriosis Treatment market witnesses a growing trend toward the integration of smart technologies. It incorporates features like remote monitoring, automated control, and real-time diagnostics, improving operational precision and safety during procedures. Advanced disconnect switches enable seamless communication with other medical devices, facilitating synchronized treatment workflows. This trend reflects the broader healthcare shift toward digitalization and automation. Manufacturers focus on developing user-friendly interfaces and connectivity options that support hospital IT infrastructure. Such innovations help reduce manual errors and improve response times during critical interventions. The market adapts swiftly to technological advancements that enhance clinical outcomes.
- For instance, Health Canada’s October 2023 approval of MYFEMBREE marked expansion into a new national market of over 15 million women of reproductive age, reinforcing opportunities for device integration in upgraded healthcare systems.
Demand for Compact and High-Performance Disconnect Switches Increases
Compactness combined with high performance drives the design trends in for endometriosis treatment devices. It responds to space constraints in modern medical equipment and the need for reliable power management. Smaller, lightweight switches that do not compromise durability or functionality attract widespread adoption. This trend encourages manufacturers to innovate materials and miniaturize components without sacrificing safety. High electrical load capacity and quick disconnect features remain critical to meeting clinical demands. Hospitals and clinics prefer switches that fit into compact systems while ensuring operational efficiency. The market evolves with a focus on balancing size, reliability, and performance.
- For instance, the FingerTech Robotics Mini Power Switch measures just 12.7 × 12.7 × 6.35 mm and weighs only 2.15 grams, while sustaining current loads of 40 A and enduring brief bursts up to 100 A.
Emphasis on Compliance with Evolving Regulatory Standards
Regulatory compliance continues to shape trends in the Endometriosis Treatment market. It drives the adoption of disconnect switches that meet increasingly stringent safety, electromagnetic compatibility, and environmental regulations. Manufacturers prioritize certifications that align with global healthcare standards, enhancing market credibility. This focus prompts continuous improvements in product testing, documentation, and quality control processes. Regulatory changes push the industry toward higher transparency and traceability of components. Healthcare providers seek devices that guarantee patient and operator safety throughout treatment procedures. Compliance-related trends ensure sustained trust and acceptance in the market.
Growing Focus on Sustainable and Eco-Friendly Solutions
Sustainability gains traction as a key trend influencing the Endometriosis Treatment market. It encourages the development of eco-friendly materials and energy-efficient designs in disconnect switches. Manufacturers adopt green manufacturing practices to reduce environmental impact and support corporate responsibility goals. Energy-saving features in disconnect switches contribute to lowering the overall power consumption of medical devices. Hospitals increasingly value products with reduced carbon footprints and compliance with environmental regulations. This trend aligns with the global push toward sustainable healthcare infrastructure. The market responds by integrating sustainability into product innovation and lifecycle management.
Market Challenges Analysis
High Costs and Complex Integration Impede Market Expansion
The Endometriosis Treatment market faces challenges related to high production and implementation costs. Advanced disconnect switches often require sophisticated materials and precise manufacturing processes, increasing their price. It limits adoption, especially among smaller healthcare providers with constrained budgets. Complex integration with existing medical equipment also poses difficulties, requiring specialized technical expertise. Hospitals and clinics may hesitate to upgrade systems due to compatibility concerns and potential downtime. These factors slow the pace of market penetration despite growing demand. Overcoming cost and integration barriers remains critical for broader acceptance.
Stringent Regulatory Requirements and Safety Concerns Limit Flexibility
Strict regulatory frameworks governing medical device components create challenges for the Endometriosis Treatment market. It must comply with rigorous safety, electromagnetic compatibility, and environmental standards. The certification process involves extensive testing, documentation, and validation, extending product development timelines. Manufacturers face pressure to maintain consistent quality while adapting to changing regulations. Safety concerns also demand robust design features to prevent malfunctions during delicate endometriosis treatments. These constraints reduce flexibility in innovation and increase operational costs. Navigating regulatory complexities requires careful planning and resource allocation.
Market Opportunities
Advancements in Medical Device Technology Create Growth Potential
The Endometriosis Treatment market can leverage rapid advancements in medical device technology to expand its footprint. It benefits from innovations that improve precision, automation, and real-time control in treatment equipment. Integration of IoT-enabled disconnect switches allows remote diagnostics and predictive maintenance, increasing operational efficiency for healthcare facilities. Manufacturers can explore miniaturization and advanced materials to meet the demand for compact yet durable components. These developments open opportunities to serve high-performance requirements in modern medical environments. Strategic collaborations with medical device makers can further strengthen market positioning. The alignment with cutting-edge healthcare technology presents a pathway for sustained growth.
Rising Healthcare Investments and Emerging Market Adoption
Increasing healthcare investments worldwide create favorable conditions for the Endometriosis Treatment market. It gains momentum from the expansion of hospital infrastructure, particularly in developing economies with growing access to specialized women’s health services. Emerging markets offer untapped potential due to rising awareness and improved affordability of advanced treatment solutions. Manufacturers can target these regions with cost-optimized products that maintain regulatory compliance. Partnerships with local distributors and healthcare providers can accelerate market entry. Enhanced funding for women’s health initiatives also supports product demand. These factors position the market for long-term expansion in diverse geographic regions.
Market Segmentation Analysis:
By Disease Type:
The Endometriosis Treatment market addresses diverse disease manifestations, with segment differentiation based on the type of endometriosis. Superficial peritoneal endometriosis cases require consistent device safety and reliability during minimally invasive procedures. It supports treatment systems designed for delicate tissue management in these cases. Ovarian endometriomas present more complex requirements, where disconnect switches must ensure precise control over power flow to surgical and diagnostic devices. Deep infiltrating endometriosis often involves intricate surgical interventions, demanding high-performance switches capable of sustaining operational stability under prolonged use. The segment’s growth reflects increasing clinical adoption of specialized devices across all disease categories.
- For instance, a multicenter French study recorded 1,135 cases of colorectal deep infiltrating endometriosis surgeries in one year, highlighting the volume and complexity of procedures requiring dependable device functionality.The study, conducted across 56 French healthcare facilities, focused on surgeries involving the rectum and sigmoid colon.
By Treatment Type:
Treatment type segmentation highlights distinct applications for treatment integration. Hormone therapy remains a first-line approach for managing endometriosis symptoms, with device usage primarily in diagnosis and monitoring. It benefits from disconnect switches that ensure uninterrupted function in hormone delivery systems and diagnostic tools. Pain medication treatments involve supportive devices for pain management procedures, requiring reliable switch performance to maintain safety during use. The adaptability of disconnect switch designs to both pharmaceutical and procedural treatment environments broadens market relevance across healthcare settings.
- For instance, Brigham and Women’s Hospital reported that MRI achieved a sensitivity of 88.3% for detecting colorectal endometriosis, underlining the precision needed in diagnostic-linked device support.
By Drug Class:
Segmentation by drug class reveals varied operational contexts for Treatment. Gonadotropin-releasing hormone therapies rely on precision-controlled delivery systems, where it plays a role in safeguarding against electrical or operational faults. NSAID treatments are often supported by diagnostic or monitoring devices, requiring dependable power control mechanisms. Oral contraceptive-based treatments involve long-term patient management, with associated medical equipment needing stable and safe operation. This diversity in drug class applications creates consistent demand for disconnect switches that can adapt to multiple device configurations while meeting strict safety standards.
Segments:
Based on Disease Type:
- Superficial peritoneal endometriosis
- Ovarian endometriomas
- Deep infiltrating endometriosis
Based on Treatment Type:
- Hormone therapy
- Pain medication
Based on Drug Class:
- Gonadotropin releasing hormone
- NSAIDs
- Oral contraceptive
Based on the Geography:
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis
North America
North America holds the largest share of the Endometriosis Treatment market at 38%, driven by advanced healthcare infrastructure and high adoption rates of innovative medical technologies. The region benefits from strong regulatory frameworks, such as the FDA’s rigorous standards, which ensure product quality and safety. It supports consistent investment in specialized medical equipment, including disconnect switches designed for endometriosis treatment devices. Leading manufacturers actively collaborate with hospitals and research institutions to integrate next-generation features like IoT connectivity and automated safety controls. The high prevalence of endometriosis in the United States and Canada contributes to steady demand for precise and reliable device components. Government-backed women’s health initiatives and favorable reimbursement policies further strengthen market penetration. This environment positions North America as a hub for innovation and sustained revenue growth.
Europe
Europe accounts for 27% of the Endometriosis Treatment market, supported by a robust network of healthcare providers and strict medical device regulations under the EU Medical Device Regulation (MDR). It benefits from a high level of clinical expertise and established referral systems for women’s health issues. Countries such as Germany, France, and the UK lead in the adoption of advanced surgical and diagnostic equipment, creating consistent demand for high-performance disconnect switches. The presence of global and regional manufacturers fosters competitive product development, emphasizing compact designs and energy efficiency. Increasing awareness of endometriosis, coupled with government health campaigns, drives early diagnosis and treatment adoption. Strong regulatory oversight ensures that only certified and reliable components enter the market, enhancing trust among healthcare providers. This combination of quality assurance and clinical readiness supports stable market growth across Europe.
Asia-Pacific
Asia-Pacific holds a 22% share of the Endometriosis Treatment market, with growth driven by expanding healthcare infrastructure and rising awareness of women’s health issues. It benefits from rapid technological adoption in countries such as Japan, China, and South Korea, where advanced medical equipment is increasingly integrated into treatment facilities. Emerging economies like India and Indonesia present strong potential due to improving healthcare access and rising investments in hospital modernization. Manufacturers focus on cost-effective designs to cater to price-sensitive markets while maintaining safety and compliance standards. Government initiatives to improve maternal and reproductive health create further opportunities for adoption. The region’s large patient base and growing medical tourism sector support higher demand for advanced treatment solutions. Strategic partnerships with local distributors enhance market penetration across diverse healthcare systems.
Latin America
Latin America represents 8% of the Endometriosis Treatment market, with Brazil, Mexico, and Argentina leading regional demand. It experiences gradual adoption of advanced disconnect switch technologies as healthcare systems modernize. Urban hospitals increasingly invest in reliable medical device components to improve treatment safety and efficiency. It benefits from growing private healthcare investment and partnerships with international manufacturers. Awareness campaigns targeting women’s reproductive health encourage early diagnosis, increasing the need for specialized treatment equipment. Challenges such as regulatory delays and limited rural access remain, but expanding private hospital networks mitigate these constraints. The region shows potential for steady growth with focused investment in high-quality medical devices.
Middle East & Africa
The Middle East & Africa account for 5% of the Endometriosis Treatment market, with adoption primarily concentrated in Gulf Cooperation Council (GCC) countries and South Africa. It benefits from healthcare modernization projects and increased government spending on specialized women’s health services. High-end private hospitals in the UAE, Saudi Arabia, and Qatar invest in advanced surgical and diagnostic devices, creating opportunities for high-performance disconnect switches. Awareness of endometriosis is rising, though it remains lower compared to developed regions. International collaborations and medical tourism in the Middle East support gradual market expansion. In Africa, adoption is limited to major urban centers due to infrastructure constraints, but donor-funded health programs contribute to improved access. Strategic focus on training and technology transfer can enhance regional uptake over the forecast period.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Astellas Pharma, Inc.
- AbbVie, Inc.
- Zydus Healthcare Limited
- Pfizer, Inc.
- Takeda Pharmaceutical Company Limited
- ObsEva SA
- Gedeon Richter Plc.
- Bayer AG
- Teva Pharmaceutical Industries Ltd.
- AstraZeneca
Competitive Analysis
The leading players in the Endometriosis Treatment market include Astellas Pharma, Inc., AbbVie, Inc., Pfizer, Inc., and Bayer AG. These companies maintain strong market positions through extensive product portfolios, advanced research capabilities, and strategic collaborations. They invest heavily in innovation to develop disconnect switch solutions that enhance safety, precision, and compatibility with modern medical devices used in endometriosis treatment. Competitive strategies focus on integrating smart technologies, improving energy efficiency, and meeting evolving regulatory requirements across global markets. Each company leverages its established distribution networks and long-standing relationships with healthcare providers to ensure consistent product availability. Continuous investment in clinical research and device optimization supports differentiation and customer trust. The competitive landscape is characterized by ongoing efforts to capture emerging markets through targeted pricing strategies and localized production capabilities. Regulatory compliance, quality assurance, and brand reputation remain critical factors influencing competitive advantage. The market competition also drives advancements in sustainability and eco-friendly manufacturing practices, aligning with global healthcare trends. By balancing technological innovation with operational efficiency, leading players reinforce their market share while addressing diverse clinical needs in the evolving healthcare environment.
Recent Developments
- In 2024, PHOENIX Group entered into a Reduced Wholesale Model agreement with AstraZeneca for the distribution of its portfolio of medicines, including Zoladex, in the United Kingdom. This strategic partnership aims to enhance the availability and distribution of AstraZeneca’s treatments to healthcare providers across the region.
- In January 2023, ObsEva reported additional positive efficacy results from the same Phase 3 trial EDELWEISS 3, confirming rapid onset and sustained pain reduction, supporting long-term treatment potential with linzagolix.
- In June 2022, the European Commission granted marketing authorization for Linzagolix (Yselty®), an oral GnRH antagonist for moderate-to-severe uterine fibroid symptoms and expanded approval for endometriosis-associated pain.
Market Concentration & Characteristics
The Endometriosis Treatment market exhibits a moderately concentrated structure, with a few multinational companies holding significant influence through extensive product portfolios and strong distribution networks. It is characterized by high entry barriers due to stringent regulatory requirements, advanced manufacturing capabilities, and the need for specialized technical expertise. Established players dominate through continuous investment in innovation, focusing on compact designs, energy efficiency, and integration with advanced medical devices. The market emphasizes safety, reliability, and compliance with global healthcare standards, driving consistent demand for certified components. Competition encourages differentiation through technology upgrades, strategic partnerships, and regional expansion strategies. It demonstrates steady growth potential, supported by increasing healthcare infrastructure investments, rising awareness of women’s health, and the adoption of advanced treatment technologies in both developed and emerging economies.
Report Coverage
The research report offers an in-depth analysis based on Disease Type, Treatment Type, Drug class and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market is expected to witness steady growth driven by rising prevalence of endometriosis and demand for advanced treatment equipment.
- Technological innovations will focus on compact, energy-efficient, and IoT-enabled disconnect switch designs.
- Integration with automated medical systems will enhance operational safety and precision during treatment procedures.
- Regulatory compliance will remain a key factor shaping product development and market access.
- Manufacturers will expand their presence in emerging economies through localized production and distribution networks.
- Sustainability will gain importance, with increased use of eco-friendly materials and green manufacturing practices.
- Strategic partnerships between medical device manufacturers and healthcare providers will strengthen adoption.
- Increased investment in women’s health initiatives will create new opportunities for product integration.
- Growing medical tourism in developing regions will contribute to higher demand for advanced treatment solutions.
- Continuous research and development will drive innovation, improving device compatibility and performance standards.




