CHAPTER NO. 1 : INTRODUCTION 24
1.1.1. Report Description 24
Purpose of the Report 24
USP & Key Offerings 24
1.1.2. Key Benefits for Stakeholders 24
1.1.3. Target Audience 25
1.1.4. Report Scope 25
1.1.5. Regional Scope 26
CHAPTER NO. 2 : EXECUTIVE SUMMARY 27
2.1. CLINICAL TRIAL MANAGEMENT SYSTEM Market Snapshot 27
2.1.1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market, 2018 – 2032 (USD Million) 28
CHAPTER NO. 3 : GEOPOLITICAL CRISIS IMPACT ANALYSIS 29
3.1. Russia-Ukraine and Israel-Palestine War Impacts 29
CHAPTER NO. 4 : CLINICAL TRIAL MANAGEMENT SYSTEM MARKET – INDUSTRY ANALYSIS 30
4.1. Introduction 30
4.2. Market Drivers 31
4.2.1. Driving Factor 1 Analysis 31
4.2.2. Driving Factor 2 Analysis 32
4.3. Market Restraints 33
4.3.1. Restraining Factor Analysis 33
4.4. Market Opportunities 34
4.4.1. Market Opportunity Analysis 34
4.5. Porter’s Five Forces Analysis 35
4.6. Value Chain Analysis 36
4.7. Buying Criteria 37
CHAPTER NO. 5 : IMPORT EXPORT ANALYSIS 38
5.1. Import Analysis by Region 38
5.1.1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Import Volume/Revenue, By Region, 2018 – 2023 38
5.2. Export Analysis by Region 39
5.2.1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Export Volume/Revenue, By Region, 2018 – 2023 39
CHAPTER NO. 6 : DEMAND SUPPLY ANALYSIS 40
6.1. Demand Analysis by Region 40
6.1.1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Demand Volume/Revenue, By Region, 2018 – 2023 40
6.2. Supply Analysis by Region 41
6.2.1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Supply Volume/Revenue, By Region, 2018 – 2023 41
CHAPTER NO. 7 : PRODUCTION ANALYSIS 42
7.1. Production Analysis by Region 42
7.1.1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Production Volume/Revenue, By Region, 2018 – 2023 42
CHAPTER NO. 8 : PRICE ANALYSIS 43
8.1. Price Analysis by Region 43
8.1.1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Price, By Region, 2018 – 2023 43
8.1.2. Europe Deployment Market Price, By Region, 2018 – 2023 43
8.2. Price Analysis by Deployment 44
8.2.1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Price, By Deployment, 2018 – 2023 44
8.2.2. Europe Deployment Market Price, By Deployment, 2018 – 2023 44
CHAPTER NO. 9 : RAW MATERIALS ANALYSIS 45
9.1. Key Raw Materials and Suppliers 45
9.2. Key Raw Materials Price Trend 45
CHAPTER NO. 10 : MANUFACTURING COST ANALYSIS 46
10.1. Manufacturing Cost Analysis 46
10.2. Manufacturing Process 46
CHAPTER NO. 11 : ANALYSIS COMPETITIVE LANDSCAPE 47
11.1. Company Market Share Analysis – 2023 47
11.1.1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market: Company Market Share, by Volume, 2023 47
11.1.2. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market: Company Market Share, by Revenue, 2023 48
11.1.3. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market: Top 6 Company Market Share, by Revenue, 2023 48
11.1.4. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market: Top 3 Company Market Share, by Revenue, 2023 49
11.2. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Company Volume Market Share, 2023 50
11.3. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Company Revenue Market Share, 2023 51
11.4. Company Assessment Metrics, 2023 52
11.4.1. Stars 52
11.4.2. Emerging Leaders 52
11.4.3. Pervasive Players 52
11.4.4. Participants 52
11.5. Start-ups /SMEs Assessment Metrics, 2023 52
11.5.1. Progressive Companies 52
11.5.2. Responsive Companies 52
11.5.3. Dynamic Companies 52
11.5.4. Starting Blocks 52
11.6. Strategic Developments 53
11.6.1. Acquisitions & Mergers 53
New Product Launch 53
Regional Expansion 53
11.7. Key Players Product Matrix 54
CHAPTER NO. 12 : PESTEL & ADJACENT MARKET ANALYSIS 55
12.1. PESTEL 55
12.1.1. Political Factors 55
12.1.2. Economic Factors 55
12.1.3. Social Factors 55
12.1.4. Technological Factors 55
12.1.5. Environmental Factors 55
12.1.6. Legal Factors 55
12.2. Adjacent Market Analysis 55
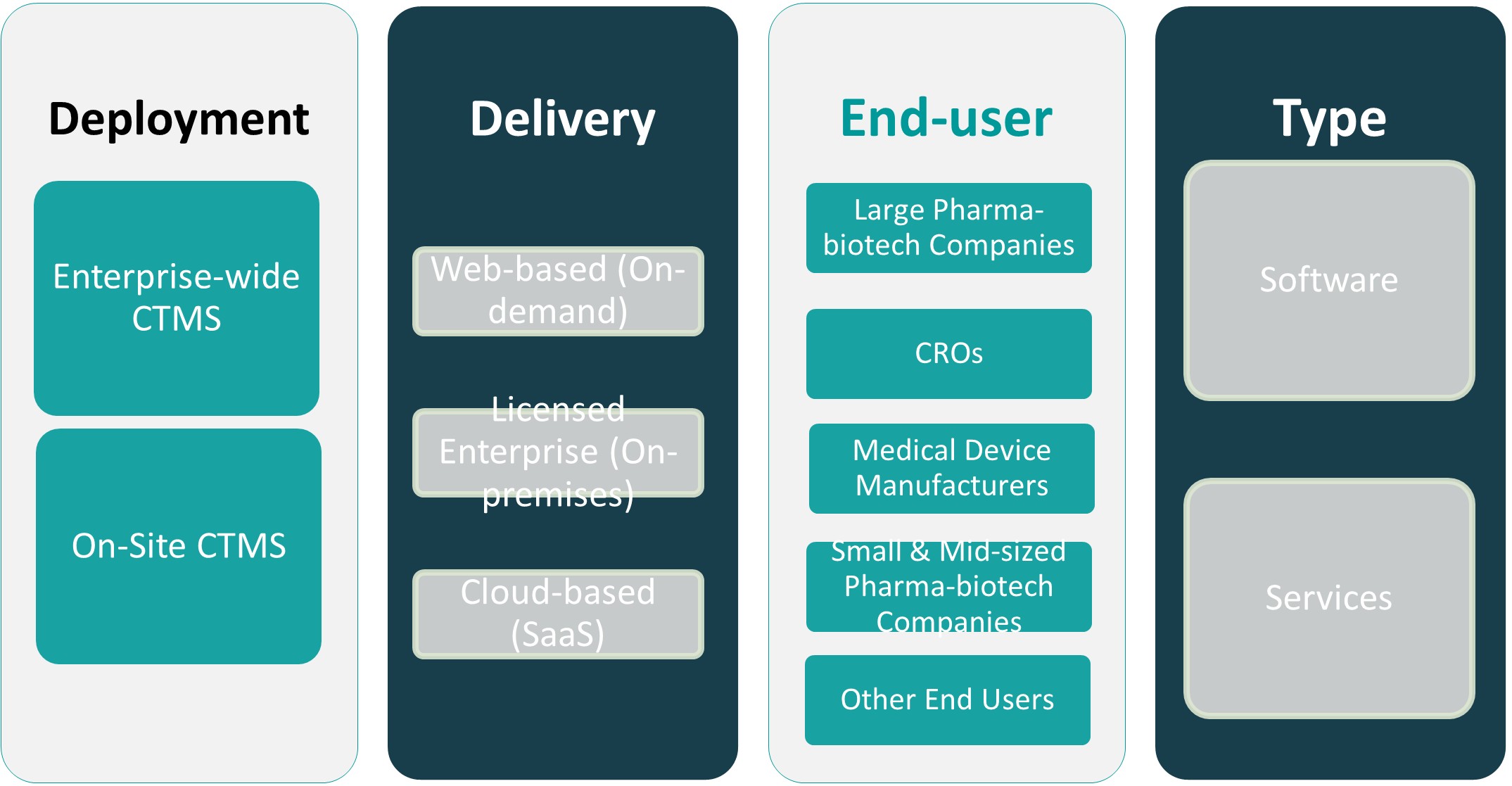
CHAPTER NO. 13 : CLINICAL TRIAL MANAGEMENT SYSTEM MARKET – BY DEPLOYMENT SEGMENT ANALYSIS 56
13.1. CLINICAL TRIAL MANAGEMENT SYSTEM Market Overview, by Deployment Segment 56
13.1.1. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue Share, By Deployment, 2023 & 2032 57
13.1.2. CLINICAL TRIAL MANAGEMENT SYSTEM Market Attractiveness Analysis, By Deployment 58
13.1.3. Incremental Revenue Growth Opportunity, by Deployment, 2024 – 2032 58
13.1.4. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018, 2023, 2027 & 2032 59
13.2. Enterprise-wide CTMS 60
13.2.1. Europe Enterprise-wide CTMS CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 61
13.2.2. Europe Enterprise-wide CTMS CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 61
13.3. On-Site CTMS 62
13.3.1. Europe On-Site CTMS CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 63
13.3.2. Europe On-Site CTMS CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 63
13.4. Deployment 3 64
13.4.1. Europe Deployment 3 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 65
13.4.2. Europe Deployment 3 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 65
13.5. Deployment 4 66
13.5.1. Europe Deployment 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 67
13.5.2. Europe Deployment 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 67
13.6. Deployment 5 68
13.6.1. Europe Deployment 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 69
13.6.2. Europe Deployment 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 69
CHAPTER NO. 14 : CLINICAL TRIAL MANAGEMENT SYSTEM MARKET – BY DELIVERY SEGMENT ANALYSIS 70
14.1. CLINICAL TRIAL MANAGEMENT SYSTEM Market Overview, by Delivery Segment 70
14.1.1. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue Share, By Delivery, 2023 & 2032 71
14.1.2. CLINICAL TRIAL MANAGEMENT SYSTEM Market Attractiveness Analysis, By Delivery 72
14.1.3. Incremental Revenue Growth Opportunity, by Delivery, 2024 – 2032 72
14.1.4. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018, 2023, 2027 & 2032 73
14.2. Web-based (On-demand) 74
14.2.1. Europe Web-based (On-demand) CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 75
14.2.2. Europe Web-based (On-demand) CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 75
14.3. Licensed Enterprise (On-premises) 76
14.3.1. Europe Licensed Enterprise (On-premises) CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 77
14.3.2. Europe Licensed Enterprise (On-premises) CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 77
14.4. Cloud-based (SaaS) 78
14.4.1. Europe Cloud-based (SaaS) CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 79
14.4.2. Europe Cloud-based (SaaS) CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 79
14.5. Delivery 4 80
14.5.1. Europe Delivery 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 81
14.5.2. Europe Delivery 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 81
14.6. Delivery 5 82
14.6.1. Europe Delivery 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 83
14.6.2. Europe Delivery 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 83
CHAPTER NO. 15 : CLINICAL TRIAL MANAGEMENT SYSTEM MARKET – BY END-USER SEGMENT ANALYSIS 84
15.1. CLINICAL TRIAL MANAGEMENT SYSTEM Market Overview, by End-user Segment 84
15.1.1. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue Share, By End-user, 2023 & 2032 85
15.1.2. CLINICAL TRIAL MANAGEMENT SYSTEM Market Attractiveness Analysis, By End-user 86
15.1.3. Incremental Revenue Growth Opportunity, by End-user, 2024 – 2032 86
15.1.4. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018, 2023, 2027 & 2032 87
15.2. Large Pharma-biotech Companies 88
15.2.1. Europe Large Pharma-biotech Companies CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 89
15.2.2. Europe Large Pharma-biotech Companies CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 89
15.3. CROs 90
15.3.1. Europe CROs CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 91
15.3.2. Europe CROs CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 91
15.4. Medical Device Manufacturers 92
15.4.1. Europe Medical Device Manufacturers CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 93
15.4.2. Europe Medical Device Manufacturers CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 93
15.5. Small & Mid-sized Pharma-biotech Companies 94
15.5.1. Europe Small & Mid-sized Pharma-biotech Companies CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 95
15.5.2. Europe Small & Mid-sized Pharma-biotech Companies CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 95
15.6. Other End Users 96
15.6.1. Europe Other End Users CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 97
15.6.2. Europe Other End Users CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 97
CHAPTER NO. 16 : CLINICAL TRIAL MANAGEMENT SYSTEM MARKET – BY TYPE SEGMENT ANALYSIS 98
16.1. CLINICAL TRIAL MANAGEMENT SYSTEM Market Overview, by Type Segment 98
16.1.1. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue Share, By Type, 2023 & 2032 99
16.1.2. CLINICAL TRIAL MANAGEMENT SYSTEM Market Attractiveness Analysis, By Type 100
16.1.3. Incremental Revenue Growth Opportunity, by Type, 2024 – 2032 100
16.1.4. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018, 2023, 2027 & 2032 101
16.2. Software 102
16.2.1. Europe Software CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 103
16.2.2. Europe Software CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 103
16.3. Services 104
16.3.1. Europe Services CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 105
16.3.2. Europe Services CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 105
16.4. Type 3 106
16.4.1. Europe Type 3 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 107
16.4.2. Europe Type 3 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 107
16.5. Type 4 108
16.5.1. Europe Type 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 109
16.5.2. Europe Type 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 109
16.6. Type 5 110
16.6.1. Europe Type 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 111
16.6.2. Europe Type 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 111
CHAPTER NO. 17 : CLINICAL TRIAL MANAGEMENT SYSTEM MARKET – BY DISTRIBUTION CHANNEL SEGMENT ANALYSIS 112
17.1. CLINICAL TRIAL MANAGEMENT SYSTEM Market Overview, by Distribution Channel Segment 112
17.1.1. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue Share, By Distribution Channel, 2023 & 2032 113
17.1.2. CLINICAL TRIAL MANAGEMENT SYSTEM Market Attractiveness Analysis, By Distribution Channel 114
17.1.3. Incremental Revenue Growth Opportunity, by Distribution Channel, 2024 – 2032 114
17.1.4. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018, 2023, 2027 & 2032 115
17.2. Distribution Channel 1 116
17.2.1. Europe Distribution Channel 1 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 117
17.2.2. Europe Distribution Channel 1 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 117
17.3. Distribution Channel 2 118
17.3.1. Europe Distribution Channel 2 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 119
17.3.2. Europe Distribution Channel 2 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 119
17.4. Distribution Channel 3 120
17.4.1. Europe Distribution Channel 3 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 121
17.4.2. Europe Distribution Channel 3 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 121
17.5. Distribution Channel 4 122
17.5.1. Europe Distribution Channel 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 123
17.5.2. Europe Distribution Channel 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 123
17.6. Distribution Channel 5 124
17.6.1. Europe Distribution Channel 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 125
17.6.2. Europe Distribution Channel 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 125
CHAPTER NO. 18 : CLINICAL TRIAL MANAGEMENT SYSTEM MARKET – REGIONAL ANALYSIS 126
18.1. CLINICAL TRIAL MANAGEMENT SYSTEM Market Overview, by Regional Segments 126
18.2. Region 127
18.2.1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue Share, By Region, 2023 & 2032 127
18.2.2. CLINICAL TRIAL MANAGEMENT SYSTEM Market Attractiveness Analysis, By Region 128
18.2.3. Incremental Revenue Growth Opportunity, by Region, 2024 – 2032 128
18.2.4. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018, 2023, 2027 & 2032 129
18.2.5. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 130
18.2.6. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 130
18.3. Deployment 131
18.3.1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018 – 2023 (USD Million) 131
18.4. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2024 – 2032 (USD Million) 131
18.5. Delivery 132
18.5.1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018 – 2023 (USD Million) 132
18.5.2. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2024 – 2032 (USD Million) 132
18.6. End-user 133
18.6.1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018 – 2023 (USD Million) 133
18.6.2. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2024 – 2032 (USD Million) 133
18.7. Type 134
18.7.1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018 – 2023 (USD Million) 134
18.7.2. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2024 – 2032 (USD Million) 134
18.8. Distribution Channel 135
18.8.1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018 – 2023 (USD Million) 135
18.8.2. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2024 – 2032 (USD Million) 135
CHAPTER NO. 19 : CLINICAL TRIAL MANAGEMENT SYSTEM MARKET – NORTH AMERICA 136
19.1. North America 136
19.1.1. Key Highlights 136
19.1.2. North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2018 – 2023 (USD Million) 137
19.1.3. North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018 – 2023 (USD Million) 138
19.1.4. North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018 – 2023 (USD Million) 139
19.1.5. North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018 – 2023 (USD Million) 140
19.1.6. North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018 – 2023 (USD Million) 141
19.1.7. North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018 – 2023 (USD Million) 142
19.2. U.S. 143
19.3. Canada 143
19.4. Mexico 143
CHAPTER NO. 20 : CLINICAL TRIAL MANAGEMENT SYSTEM MARKET – EUROPE 144
20.1. Europe 144
20.1.1. Key Highlights 144
20.1.2. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2018 – 2023 (USD Million) 145
20.1.3. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018 – 2023 (USD Million) 146
20.1.4. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018 – 2023 (USD Million) 147
20.1.5. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018 – 2023 (USD Million) 148
20.1.6. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018 – 2023 (USD Million) 149
20.1.7. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018 – 2023 (USD Million) 150
20.2. UK 151
20.3. France 151
20.4. Germany 151
20.5. Italy 151
20.6. Spain 151
20.7. Russia 151
20.8. Belgium 151
20.9. Netherland 151
20.10. Austria 151
20.11. Sweden 151
20.12. Poland 151
20.13. Denmark 151
20.14. Switzerland 151
20.15. Rest of Europe 151
CHAPTER NO. 21 : CLINICAL TRIAL MANAGEMENT SYSTEM MARKET – ASIA PACIFIC 152
21.1. Asia Pacific 152
21.1.1. Key Highlights 152
21.1.2. Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2018 – 2023 (USD Million) 153
21.1.3. Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018 – 2023 (USD Million) 154
21.1.4. Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018 – 2023 (USD Million) 155
21.1.5. Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018 – 2023 (USD Million) 156
21.1.6. Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018 – 2023 (USD Million) 157
21.1.7. Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018 – 2023 (USD Million) 158
21.2. China 159
21.3. Japan 159
21.4. South Korea 159
21.5. India 159
21.6. Australia 159
21.7. Thailand 159
21.8. Indonesia 159
21.9. Vietnam 159
21.10. Malaysia 159
21.11. Philippines 159
21.12. Taiwan 159
21.13. Rest of Asia Pacific 159
CHAPTER NO. 22 : CLINICAL TRIAL MANAGEMENT SYSTEM MARKET – LATIN AMERICA 160
22.1. Latin America 160
22.1.1. Key Highlights 160
22.1.2. Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2018 – 2023 (USD Million) 161
22.1.3. Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018 – 2023 (USD Million) 162
22.1.4. Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018 – 2023 (USD Million) 163
22.1.5. Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018 – 2023 (USD Million) 164
22.1.6. Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018 – 2023 (USD Million) 165
22.1.7. Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018 – 2023 (USD Million) 166
22.2. Brazil 167
22.3. Argentina 167
22.4. Peru 167
22.5. Chile 167
22.6. Colombia 167
22.7. Rest of Latin America 167
CHAPTER NO. 23 : CLINICAL TRIAL MANAGEMENT SYSTEM MARKET – MIDDLE EAST 168
23.1. Middle East 168
23.1.1. Key Highlights 168
23.1.2. Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2018 – 2023 (USD Million) 169
23.1.3. Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018 – 2023 (USD Million) 170
23.1.4. Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018 – 2023 (USD Million) 171
23.1.5. Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018 – 2023 (USD Million) 172
23.1.6. Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018 – 2023 (USD Million) 173
23.1.7. Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018 – 2023 (USD Million) 174
23.2. UAE 175
23.3. KSA 175
23.4. Israel 175
23.5. Turkey 175
23.6. Iran 175
23.7. Rest of Middle East 175
CHAPTER NO. 24 : CLINICAL TRIAL MANAGEMENT SYSTEM MARKET – AFRICA 176
24.1. Africa 176
24.1.1. Key Highlights 176
24.1.2. Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2018 – 2023 (USD Million) 177
24.1.3. Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018 – 2023 (USD Million) 178
24.1.4. Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018 – 2023 (USD Million) 179
24.1.5. Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018 – 2023 (USD Million) 180
24.1.6. Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018 – 2023 (USD Million) 181
24.1.7. Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018 – 2023 (USD Million) 182
24.2. Egypt 183
24.3. Nigeria 183
24.4. Algeria 183
24.5. Morocco 183
24.6. Rest of Africa 183
CHAPTER NO. 25 : COMPANY PROFILES 184
25.1. Forte Research Systems 184
25.1.1. Company Overview 184
25.1.2. Product Portfolio 184
25.1.3. Swot Analysis 184
25.1.4. Business Strategy 185
25.1.5. Financial Overview 185
25.1.6. ICON plc 186
25.1.7. Merge healthcare incorporated 186
25.1.8. Bio-Optronics 186
25.1.9. DSG INC 186
25.1.10. ArisEurope 186
25.1.11. ERT Clinical Bioclinica 186
25.1.12. Oracle Corporation 186
25.1.13. Medidata Solutions 186
25.1.14. DATATRAK International, Inc 186
25.1.15. Company 11 186
25.1.16. Company 12 186
25.1.17. Company 13 186
25.1.18. Company 14 186
CHAPTER NO. 26 : RESEARCH METHODOLOGY 187
26.1. Research Methodology 187
26.1.1. Phase I – Secondary Research 188
26.1.2. Phase II – Data Modeling 188
Company Share Analysis Model 189
Revenue Based Modeling 189
26.1.3. Phase III – Primary Research 190
26.1.4. Research Limitations 191
Assumptions 191
List of Figures
FIG NO. 1. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, 2018 – 2032 (USD Million) 30
FIG NO. 2. Porter’s Five Forces Analysis for Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market 37
FIG NO. 3. Value Chain Analysis for Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market 38
FIG NO. 4. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Import Volume/Revenue, By Region, 2018 – 2023 40
FIG NO. 5. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Export Volume/Revenue, By Region, 2018 – 2023 41
FIG NO. 6. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Demand Volume/Revenue, By Region, 2018 – 2023 42
FIG NO. 7. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Supply Volume/Revenue, By Region, 2018 – 2023 43
FIG NO. 8. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Production Volume/Revenue, By Region, 2018 – 2023 44
FIG NO. 9. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Price, By Region, 2018 – 2023 45
FIG NO. 10. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Price, By Deployment, 2018 – 2023 46
FIG NO. 11. Raw Materials Price Trend Analysis, 2018 – 2023 47
FIG NO. 12. Manufacturing Cost Analysis 48
FIG NO. 13. Manufacturing Process 48
FIG NO. 14. Company Share Analysis, 2023 49
FIG NO. 15. Company Share Analysis, 2023 50
FIG NO. 16. Company Share Analysis, 2023 50
FIG NO. 17. Company Share Analysis, 2023 51
FIG NO. 18. CLINICAL TRIAL MANAGEMENT SYSTEM Market – Company Volume Market Share, 2023 52
FIG NO. 19. CLINICAL TRIAL MANAGEMENT SYSTEM Market – Company Revenue Market Share, 2023 53
FIG NO. 20. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue Share, By Deployment, 2023 & 2032 59
FIG NO. 21. Market Attractiveness Analysis, By Deployment 60
FIG NO. 22. Incremental Revenue Growth Opportunity by Deployment, 2024 – 2032 60
FIG NO. 23. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018, 2023, 2027 & 2032 61
FIG NO. 24. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Enterprise-wide CTMS, Revenue (USD Million) 2018 – 2032 62
FIG NO. 25. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for On-Site CTMS, Revenue (USD Million) 2018 – 2032 64
FIG NO. 26. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Deployment 3, Revenue (USD Million) 2018 – 2032 66
FIG NO. 27. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Deployment 4, Revenue (USD Million) 2018 – 2032 68
FIG NO. 28. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Deployment 5, Revenue (USD Million) 2018 – 2032 70
FIG NO. 29. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue Share, By Delivery, 2023 & 2032 73
FIG NO. 30. Market Attractiveness Analysis, By Delivery 74
FIG NO. 31. Incremental Revenue Growth Opportunity by Delivery, 2024 – 2032 74
FIG NO. 32. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018, 2023, 2027 & 2032 75
FIG NO. 33. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Web-based (On-demand), Revenue (USD Million) 2018 – 2032 76
FIG NO. 34. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Licensed Enterprise (On-premises), Revenue (USD Million) 2018 – 2032 78
FIG NO. 35. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Cloud-based (SaaS), Revenue (USD Million) 2018 – 2032 80
FIG NO. 36. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Delivery 4, Revenue (USD Million) 2018 – 2032 82
FIG NO. 37. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Delivery 5, Revenue (USD Million) 2018 – 2032 84
FIG NO. 38. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue Share, By End-user, 2023 & 2032 87
FIG NO. 39. Market Attractiveness Analysis, By End-user 88
FIG NO. 40. Incremental Revenue Growth Opportunity by End-user, 2024 – 2032 88
FIG NO. 41. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018, 2023, 2027 & 2032 89
FIG NO. 42. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Large Pharma-biotech Companies, Revenue (USD Million) 2018 – 2032 90
FIG NO. 43. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for CROs, Revenue (USD Million) 2018 – 2032 92
FIG NO. 44. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Medical Device Manufacturers, Revenue (USD Million) 2018 – 2032 94
FIG NO. 45. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Small & Mid-sized Pharma-biotech Companies, Revenue (USD Million) 2018 – 2032 96
FIG NO. 46. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Other End Users, Revenue (USD Million) 2018 – 2032 98
FIG NO. 47. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue Share, By Type, 2023 & 2032 101
FIG NO. 48. Market Attractiveness Analysis, By Type 102
FIG NO. 49. Incremental Revenue Growth Opportunity by Type, 2024 – 2032 102
FIG NO. 50. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018, 2023, 2027 & 2032 103
FIG NO. 51. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Software, Revenue (USD Million) 2018 – 2032 104
FIG NO. 52. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Services, Revenue (USD Million) 2018 – 2032 106
FIG NO. 53. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Type 3, Revenue (USD Million) 2018 – 2032 108
FIG NO. 54. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Type 4, Revenue (USD Million) 2018 – 2032 110
FIG NO. 55. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Type 5, Revenue (USD Million) 2018 – 2032 112
FIG NO. 56. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue Share, By Distribution Channel, 2023 & 2032 115
FIG NO. 57. Market Attractiveness Analysis, By Distribution Channel 116
FIG NO. 58. Incremental Revenue Growth Opportunity by Distribution Channel, 2024 – 2032 116
FIG NO. 59. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018, 2023, 2027 & 2032 117
FIG NO. 60. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Distribution Channel 1, Revenue (USD Million) 2018 – 2032 118
FIG NO. 61. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Distribution Channel 2, Revenue (USD Million) 2018 – 2032 120
FIG NO. 62. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Distribution Channel 3, Revenue (USD Million) 2018 – 2032 122
FIG NO. 63. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Distribution Channel 4, Revenue (USD Million) 2018 – 2032 124
FIG NO. 64. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market for Distribution Channel 5, Revenue (USD Million) 2018 – 2032 126
FIG NO. 65. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue Share, By Region, 2023 & 2032 129
FIG NO. 66. Market Attractiveness Analysis, By Region 130
FIG NO. 67. Incremental Revenue Growth Opportunity by Region, 2024 – 2032 130
FIG NO. 68. CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018, 2023, 2027 & 2032 131
FIG NO. 69. North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, 2018 – 2032 (USD Million) 138
FIG NO. 70. Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, 2018 – 2032 (USD Million) 146
FIG NO. 71. Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, 2018 – 2032 (USD Million) 154
FIG NO. 72. Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, 2018 – 2032 (USD Million) 162
FIG NO. 73. Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, 2018 – 2032 (USD Million) 170
FIG NO. 74. Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, 2018 – 2032 (USD Million) 178
FIG NO. 75. Research Methodology – Detailed View 189
FIG NO. 76. Research Methodology 190
List of Tables
TABLE NO. 1. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market: Snapshot 27
TABLE NO. 2. : Drivers for the CLINICAL TRIAL MANAGEMENT SYSTEM Market: Impact Analysis 31
TABLE NO. 3. : Restraints for the CLINICAL TRIAL MANAGEMENT SYSTEM Market: Impact Analysis 33
TABLE NO. 4. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 43
TABLE NO. 5. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018 – 2023 44
TABLE NO. 6. : Key Raw Materials & Suppliers 45
TABLE NO. 7. : Europe Enterprise-wide CTMS CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 61
TABLE NO. 8. : Europe Enterprise-wide CTMS CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 61
TABLE NO. 9. : Europe On-Site CTMS CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 63
TABLE NO. 10. : Europe On-Site CTMS CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 63
TABLE NO. 11. : Europe Deployment 3 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 65
TABLE NO. 12. : Europe Deployment 3 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 65
TABLE NO. 13. : Europe Deployment 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 67
TABLE NO. 14. : Europe Deployment 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 67
TABLE NO. 15. : Europe Deployment 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 69
TABLE NO. 16. : Europe Deployment 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 69
TABLE NO. 17. : Europe Web-based (On-demand) CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 75
TABLE NO. 18. : Europe Web-based (On-demand) CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 75
TABLE NO. 19. : Europe Licensed Enterprise (On-premises) CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 77
TABLE NO. 20. : Europe Licensed Enterprise (On-premises) CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 77
TABLE NO. 21. : Europe Cloud-based (SaaS) CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 79
TABLE NO. 22. : Europe Cloud-based (SaaS) CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 79
TABLE NO. 23. : Europe Delivery 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 81
TABLE NO. 24. : Europe Delivery 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 81
TABLE NO. 25. : Europe Delivery 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 83
TABLE NO. 26. : Europe Delivery 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 83
TABLE NO. 27. : Europe Large Pharma-biotech Companies CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 89
TABLE NO. 28. : Europe Large Pharma-biotech Companies CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 89
TABLE NO. 29. : Europe CROs CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 91
TABLE NO. 30. : Europe CROs CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 91
TABLE NO. 31. : Europe Medical Device Manufacturers CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 93
TABLE NO. 32. : Europe Medical Device Manufacturers CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 93
TABLE NO. 33. : Europe Small & Mid-sized Pharma-biotech Companies CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 95
TABLE NO. 34. : Europe Small & Mid-sized Pharma-biotech Companies CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 95
TABLE NO. 35. : Europe Other End Users CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 97
TABLE NO. 36. : Europe Other End Users CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 97
TABLE NO. 37. : Europe Software CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 103
TABLE NO. 38. : Europe Software CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 103
TABLE NO. 39. : Europe Services CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 105
TABLE NO. 40. : Europe Services CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 105
TABLE NO. 41. : Europe Type 3 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 107
TABLE NO. 42. : Europe Type 3 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 107
TABLE NO. 43. : Europe Type 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 109
TABLE NO. 44. : Europe Type 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 109
TABLE NO. 45. : Europe Type 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 111
TABLE NO. 46. : Europe Type 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 111
TABLE NO. 47. : Europe Distribution Channel 1 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 117
TABLE NO. 48. : Europe Distribution Channel 1 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 117
TABLE NO. 49. : Europe Distribution Channel 2 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 119
TABLE NO. 50. : Europe Distribution Channel 2 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 119
TABLE NO. 51. : Europe Distribution Channel 3 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 121
TABLE NO. 52. : Europe Distribution Channel 3 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 121
TABLE NO. 53. : Europe Distribution Channel 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 123
TABLE NO. 54. : Europe Distribution Channel 4 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 123
TABLE NO. 55. : Europe Distribution Channel 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 125
TABLE NO. 56. : Europe Distribution Channel 5 CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 125
TABLE NO. 57. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2018 – 2023 (USD Million) 130
TABLE NO. 58. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Region, 2024 – 2032 (USD Million) 130
TABLE NO. 59. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018 – 2023 (USD Million) 131
TABLE NO. 60. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2024 – 2032 (USD Million) 131
TABLE NO. 61. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018 – 2023 (USD Million) 132
TABLE NO. 62. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2024 – 2032 (USD Million) 132
TABLE NO. 63. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018 – 2023 (USD Million) 133
TABLE NO. 64. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2024 – 2032 (USD Million) 133
TABLE NO. 65. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018 – 2023 (USD Million) 134
TABLE NO. 66. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2024 – 2032 (USD Million) 134
TABLE NO. 67. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018 – 2023 (USD Million) 135
TABLE NO. 68. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2024 – 2032 (USD Million) 135
TABLE NO. 69. : North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2018 – 2023 (USD Million) 137
TABLE NO. 70. : North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2024 – 2032 (USD Million) 137
TABLE NO. 71. : North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018 – 2023 (USD Million) 138
TABLE NO. 72. : North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2024 – 2032 (USD Million) 138
TABLE NO. 73. : North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018 – 2023 (USD Million) 139
TABLE NO. 74. : North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2024 – 2032 (USD Million) 139
TABLE NO. 75. : North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018 – 2023 (USD Million) 140
TABLE NO. 76. : North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2024 – 2032 (USD Million) 140
TABLE NO. 77. : North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018 – 2023 (USD Million) 141
TABLE NO. 78. : North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2024 – 2032 (USD Million) 141
TABLE NO. 79. : North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018 – 2023 (USD Million) 142
TABLE NO. 80. : North America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2024 – 2032 (USD Million) 142
TABLE NO. 81. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2018 – 2023 (USD Million) 145
TABLE NO. 82. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2024 – 2032 (USD Million) 145
TABLE NO. 83. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018 – 2023 (USD Million) 146
TABLE NO. 84. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2024 – 2032 (USD Million) 146
TABLE NO. 85. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018 – 2023 (USD Million) 147
TABLE NO. 86. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2024 – 2032 (USD Million) 147
TABLE NO. 87. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018 – 2023 (USD Million) 148
TABLE NO. 88. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2024 – 2032 (USD Million) 148
TABLE NO. 89. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018 – 2023 (USD Million) 149
TABLE NO. 90. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2024 – 2032 (USD Million) 149
TABLE NO. 91. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018 – 2023 (USD Million) 150
TABLE NO. 92. : Europe CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2024 – 2032 (USD Million) 150
TABLE NO. 93. : Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2018 – 2023 (USD Million) 153
TABLE NO. 94. : Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2024 – 2032 (USD Million) 153
TABLE NO. 95. : Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018 – 2023 (USD Million) 154
TABLE NO. 96. : Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2024 – 2032 (USD Million) 154
TABLE NO. 97. : Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018 – 2023 (USD Million) 155
TABLE NO. 98. : Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2024 – 2032 (USD Million) 155
TABLE NO. 99. : Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018 – 2023 (USD Million) 156
TABLE NO. 100. : Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2024 – 2032 (USD Million) 156
TABLE NO. 101. : Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018 – 2023 (USD Million) 157
TABLE NO. 102. : Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2024 – 2032 (USD Million) 157
TABLE NO. 103. : Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018 – 2023 (USD Million) 158
TABLE NO. 104. : Asia Pacific CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2024 – 2032 (USD Million) 158
TABLE NO. 105. : Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2018 – 2023 (USD Million) 161
TABLE NO. 106. : Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2024 – 2032 (USD Million) 161
TABLE NO. 107. : Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018 – 2023 (USD Million) 162
TABLE NO. 108. : Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2024 – 2032 (USD Million) 162
TABLE NO. 109. : Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018 – 2023 (USD Million) 163
TABLE NO. 110. : Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2024 – 2032 (USD Million) 163
TABLE NO. 111. : Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018 – 2023 (USD Million) 164
TABLE NO. 112. : Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2024 – 2032 (USD Million) 164
TABLE NO. 113. : Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018 – 2023 (USD Million) 165
TABLE NO. 114. : Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2024 – 2032 (USD Million) 165
TABLE NO. 115. : Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018 – 2023 (USD Million) 166
TABLE NO. 116. : Latin America CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2024 – 2032 (USD Million) 166
TABLE NO. 117. : Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2018 – 2023 (USD Million) 169
TABLE NO. 118. : Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2024 – 2032 (USD Million) 169
TABLE NO. 119. : Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018 – 2023 (USD Million) 170
TABLE NO. 120. : Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2024 – 2032 (USD Million) 170
TABLE NO. 121. : Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018 – 2023 (USD Million) 171
TABLE NO. 122. : Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2024 – 2032 (USD Million) 171
TABLE NO. 123. : Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018 – 2023 (USD Million) 172
TABLE NO. 124. : Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2024 – 2032 (USD Million) 172
TABLE NO. 125. : Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018 – 2023 (USD Million) 173
TABLE NO. 126. : Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2024 – 2032 (USD Million) 173
TABLE NO. 127. : Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018 – 2023 (USD Million) 174
TABLE NO. 128. : Middle East CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2024 – 2032 (USD Million) 174
TABLE NO. 129. : Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2018 – 2023 (USD Million) 177
TABLE NO. 130. : Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Country, 2024 – 2032 (USD Million) 177
TABLE NO. 131. : Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2018 – 2023 (USD Million) 178
TABLE NO. 132. : Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Deployment, 2024 – 2032 (USD Million) 178
TABLE NO. 133. : Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2018 – 2023 (USD Million) 179
TABLE NO. 134. : Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Delivery, 2024 – 2032 (USD Million) 179
TABLE NO. 135. : Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2018 – 2023 (USD Million) 180
TABLE NO. 136. : Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By End-user, 2024 – 2032 (USD Million) 180
TABLE NO. 137. : Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2018 – 2023 (USD Million) 181
TABLE NO. 138. : Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Type, 2024 – 2032 (USD Million) 181
TABLE NO. 139. : Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2018 – 2023 (USD Million) 182
TABLE NO. 140. : Africa CLINICAL TRIAL MANAGEMENT SYSTEM Market Revenue, By Distribution Channel, 2024 – 2032 (USD Million) 182