| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Hypoallergenic Infant Formula For CMPA Market Size 2024 |
USD 4,370.0 Million |
| Hypoallergenic Infant Formula For CMPA Market, CAGR |
8.60% |
| Hypoallergenic Infant Formula For CMPA Market Size 2032 |
USD 9,518.7 Million |
Market Overview
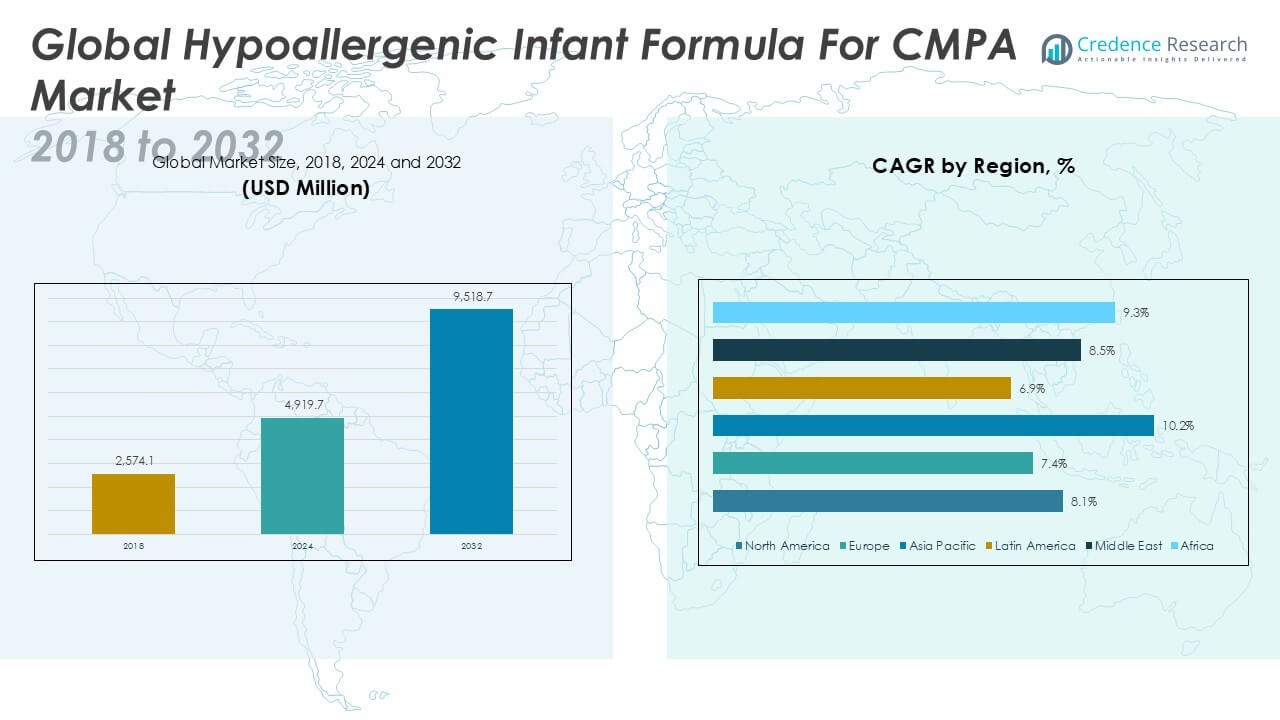
The Global Hypoallergenic Infant Formula For CMPA Market is projected to grow from USD 4,370.0 million in 2024 to an estimated USD 9,518.7 million based on 2032, with a compound annual growth rate (CAGR) of 8.60% from 2025 to 2032.
Market Drivers and Trends: The increasing prevalence of cow milk protein allergy (CMPA) among infants drives early adoption of hypoallergenic formulations. Advances in enzymatic hydrolysis and protein fractionation enhance palatability and nutrient bioavailability, boosting consumer uptake. Regulatory approvals for new variants—including partially and extensively hydrolyzed formulas and amino acid–based products—support continuous product innovation. Clean-label preferences and demand for lactose-free, organic options shape portfolio development. Direct-to-consumer subscription models and targeted digital marketing streamline access and deepen market penetration
Geographical analysis: North America leads, supported by high healthcare expenditure, robust pediatric nutrition guidelines, and established distribution networks. Asia Pacific records the fastest growth, driven by rising birth rates, growing CMPA awareness, and expanding retail access in China, India, and Southeast Asia. Europe sustains steady expansion through stringent quality standards and reimbursement schemes. Latin America and the Middle East & Africa display moderate growth trajectories. Key players shaping the competitive landscape include Nestlé, Danone, Mead Johnson (Reckitt), Abbott Laboratories, and FrieslandCampina, all of which leverage R&D investments and strategic collaborations to strengthen regional footprints
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The market is projected to grow from USD 4,370.0 million in 2024 to USD 9,518.7 million by 2032, registering a CAGR of 8.60% from 2025 to 2032.
- Increasing incidence of cow milk protein allergy (CMPA) among infants is fueling demand for specialized hypoallergenic nutrition.
- Clinical advancements in protein hydrolysis and rising adoption of amino acid-based formulas enhance product effectiveness and uptake.
- Growing consumer interest in organic, lactose-free, and clean-label infant nutrition supports premium product development.
- High product costs and limited reimbursement policies in low-income regions restrict market accessibility.
- North America holds the largest market share in 2024, while Asia Pacific is the fastest-growing region with a CAGR of 10.2%.
- Europe maintains steady growth due to strong regulatory frameworks and established pediatric nutrition protocols.
Market Drivers
Rising Incidence of Cow Milk Protein Allergy Among Infants
The Hypoallergenic Infant Formula For CMPA Market benefits from a steady increase in diagnosed CMPA cases among newborns. Pediatricians recommend hypoallergenic formulas for infants who show adverse reactions to standard milk proteins. It provides relief from gastrointestinal discomfort and supports optimal nutrient absorption. Early diagnosis methods improve detection rates and shorten the time to intervention. Caregiver education programs highlight allergy symptoms and treatment options. It drives volume growth across hospital pharmacies and retail channels. Manufacturers monitor epidemiological data to align production with emerging needs.
- For instance, protein hydrolysate-based formulas accounted for approximately 44.1% of the infant formula market by ingredient in 2023, reflecting widespread adoption for managing cow milk protein allergy in infants
Advances in Protein Hydrolysis and Formula Customization
The Hypoallergenic Infant Formula For CMPA Market incorporates cutting-edge hydrolysis techniques that break down allergenic proteins into peptides. It enhances formula tolerance without compromising essential amino acid profiles. Novel enzymatic processes refine peptide size distribution and reduce bitterness. It allows developers to tailor formulations for partially hydrolyzed and amino acid–based products. Research collaborations accelerate the introduction of formulations with improved palatability. It supports product differentiation and encourages repeat purchases.
- For instance, researchers developed an extensively hydrolyzed cow milk protein formula with a whey protein-to-casein ratio of 6:4, and in preclinical studies, this formula significantly reduced allergic markers and symptoms in animal models, demonstrating the impact of advanced hydrolysis and customization techniques.
Strengthening Regulatory Frameworks and Quality Assurance Measures
The Hypoallergenic Infant Formula For CMPA Market aligns with evolving nutrition guidelines and stringent safety standards. It undergoes rigorous clinical testing to validate hypoallergenicity and nutrient adequacy. New regulations mandate transparent labeling of protein sources and processing methods. It enhances consumer trust and reduces regulatory hurdles for market entry. Industry certifications and third-party audits reinforce compliance and quality. It fosters competitive advantage for companies that maintain exemplary safety records.
Expansion of Multichannel Distribution and Digital Outreach
The Hypoallergenic Infant Formula For CMPA Market leverages omnichannel strategies to reach caregivers and healthcare professionals. It partners with online pharmacies and telehealth platforms for convenient access. Social media campaigns educate parents on allergy management and formula selection. It integrates subscription models that ensure uninterrupted supply and build brand loyalty. Direct engagement with pediatric allergy specialists promotes product adoption. It drives sales growth through targeted digital advertising and data-driven insights.
Market Trends
Growth of Amino Acid-Based and Extensively Hydrolyzed Formulas
The Hypoallergenic Infant Formula For CMPA Market observes a shift toward amino acid-based and extensively hydrolyzed formulas due to their proven efficacy in severe allergy cases. It enables complete elimination of intact cow milk proteins, reducing allergic responses. Healthcare providers recommend these formulas for infants with complex digestive sensitivities. Manufacturers invest in advanced protein processing technologies to refine texture and taste. It improves patient compliance and strengthens brand reputation. Product lines now offer multiple variants tailored to severity levels and feeding preferences.
- For instance, the U.S. Bureau of Labor Statistics Consumer Expenditure Surveys program collected data from 490,000 submitted responses in 2023, including detailed information on household purchases of infant formula, which supports tracking the adoption of specialized formulas across large populations
Rising Preference for Organic and Clean-Label Formulations
The Hypoallergenic Infant Formula For CMPA Market reflects growing demand for organic and clean-label options. It aligns with consumer interest in natural, non-GMO, and preservative-free products. Parents prefer formulas with clearly sourced ingredients and minimal processing. Certification labels for organic and allergen-safe standards influence purchase decisions. It drives innovation in plant-based protein alternatives and lactose-free variants. Clean-label positioning contributes to premium product pricing and brand differentiation.
- For instance, the Annual Business Survey 2023 by the U.S. Census Bureau mailed surveys to 850,000 employer businesses, collecting responses on the production and sale of organic and specialty foods, including infant nutrition products, which helps quantify the scale of clean-label product offerings in the market
Integration of E-commerce and Subscription-Based Delivery Models
The Hypoallergenic Infant Formula For CMPA Market expands through online sales platforms and subscription services. It caters to the need for consistent supply among families managing CMPA. E-commerce channels offer convenience and access to a wider range of specialized formulas. Subscription models ensure regular delivery and often include personalized nutritional support. It reduces supply disruptions and builds long-term customer relationships. Brands utilize digital platforms to streamline logistics and customer engagement.
Increased Focus on Clinical Research and Pediatric Collaboration
The Hypoallergenic Infant Formula For CMPA Market benefits from extensive clinical studies and collaborations with pediatric experts. It reinforces product credibility and supports inclusion in hospital formularies. Clinical validation of hypoallergenicity helps brands secure regulatory approvals in multiple regions. Pediatric allergists and nutritionists influence consumer choices through professional recommendations. It encourages continued product refinement based on real-world evidence. Clinical research remains a cornerstone for future innovation and global expansion.
Market Challenges
High Product Costs and Limited Accessibility in Emerging Regions
The Hypoallergenic Infant Formula For CMPA Market faces challenges due to the high cost of specialized formulas. It limits affordability for middle- and low-income families, especially in developing regions. Amino acid-based and extensively hydrolyzed formulas require advanced manufacturing processes that increase production expenses. Lack of reimbursement policies in many countries restricts widespread adoption. It creates disparities in access to essential nutritional care for infants with CMPA. Price sensitivity remains a barrier to market penetration outside high-income economies.
- For instance, a clinical cost analysis in Thailand found that the direct medical cost of managing CMPA over 36 months was $1,720 for extensively hydrolyzed casein formula with probiotics, $2,791 for extensively hydrolyzed whey formula, and $7,881 for amino acid formula, highlighting the significant financial burden families may face when specialized formulas are not reimbursed.
Regulatory Complexity and Low Awareness Among Consumers
The Hypoallergenic Infant Formula For CMPA Market contends with regulatory inconsistencies across regions. It complicates product approvals and delays market entry for new formulations. Countries differ in their definitions and testing requirements for hypoallergenicity, which increases compliance costs. Low awareness of CMPA symptoms among parents leads to underdiagnosis and delayed intervention. It affects timely adoption of suitable nutritional products. Manufacturers must invest in targeted education and professional outreach to bridge the knowledge gap.
Market Opportunities
Expansion into Underserved Markets with Rising CMPA Awareness
The Hypoallergenic Infant Formula For CMPA Market has strong growth potential in underserved regions where awareness of cow milk protein allergy is increasing. It can expand through partnerships with pediatric associations and local healthcare providers. Rising birth rates and urbanization in Asia, Latin America, and Africa create a large consumer base. Government health programs and NGO initiatives focused on infant nutrition provide new distribution pathways. It allows companies to introduce cost-effective formulations tailored to local preferences. Early-stage investments in education and diagnosis support long-term market development.
Innovation in Plant-Based and Sustainable Formulation Alternatives
The Hypoallergenic Infant Formula For CMPA Market holds opportunity through innovation in plant-based protein sources and sustainable formulations. It supports consumer demand for clean-label and environmentally responsible products. R\&D into rice, pea, or oat protein bases opens avenues beyond traditional casein or whey hydrolysates. Sustainable packaging and carbon-neutral production practices improve brand positioning. It appeals to ethically conscious consumers and supports regulatory alignment with green initiatives. Companies that integrate allergen safety with sustainability can gain competitive advantage.
Market Segmentation Analysis
By Product Type
The Hypoallergenic Infant Formula For CMPA Market is segmented into Extensively Hydrolyzed Formula (eHF) and Amino Acid-Based Formula (AAF). Extensively hydrolyzed formulas hold a major volume and revenue share due to their wide use in managing moderate CMPA cases. It breaks down milk proteins into smaller peptides, reducing allergenicity while maintaining essential nutrients. Amino acid-based formulas capture a growing share, particularly for severe or complex allergy profiles. It offers complete protein elimination and is often prescribed when eHF proves ineffective. Continuous clinical validation and pediatric endorsement support the expansion of both product categories, with AAF expected to grow at a faster rate driven by rising awareness and improved formulation technologies.
- For instance, in 2023, companies reported that over 1.3 million metric tons of extensively hydrolyzed infant formula were sold globally, while amino acid-based formulas accounted for approximately 400,000 metric tons in the same year
By Distribution Channel
The Hypoallergenic Infant Formula For CMPA Market is categorized by distribution into hospital pharmacies, retail pharmacies, online retailers, and supermarkets/hypermarkets. Hospital pharmacies lead the segment in revenue due to physician-guided product recommendations and critical care requirements. Retail pharmacies maintain a strong presence through accessibility and trusted brand availability. Online retailers gain momentum due to convenience, subscription models, and increasing digital health awareness. It allows direct engagement with caregivers and supports consistent product access. Supermarkets and hypermarkets contribute to volume sales, particularly in developed regions, though with limited specialist product depth. Multi-channel strategies enhance reach and support market expansion across diverse consumer segments.
- For instance, industry surveys show that in 2024, more than 18 million units of hypoallergenic infant formula were distributed through hospital and retail pharmacies worldwide, while online retailers and supermarkets/hypermarkets accounted for over 12 million and 10 million units, respectively.
Segments
Based on Product Type
- Extensively Hydrolyzed Formula (eHF)
- Amino Acid-Based Formula (AAF)
Based on Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Retailers
- Supermarkets/Hypermarkets
Based on Region
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis
North America Hypoallergenic Infant Formula For CMPA Market
North America accounts for the largest share of the Hypoallergenic Infant Formula For CMPA Market, holding approximately 38.7% of global revenue in 2024. The market is valued at USD 1,690.9 million in 2024 and is projected to reach USD 2,903.2 million by 2032, expanding at a CAGR of 8.1%. It benefits from high awareness of CMPA, advanced diagnostic practices, and strong reimbursement frameworks. The United States leads regional consumption, supported by pediatric endorsements and widespread hospital use. E-commerce and direct-to-consumer models also enhance accessibility. It continues to grow with increased demand for premium, clinically validated formulas.
Europe Hypoallergenic Infant Formula For CMPA Market
Europe represents a significant portion of the Hypoallergenic Infant Formula For CMPA Market, contributing 30.5% of global revenue in 2024. The market stands at USD 1,331.8 million in 2024 and is expected to reach USD 2,313.0 million by 2032, growing at a CAGR of 7.4%. Countries such as Germany, France, and the UK drive growth through well-established healthcare systems and allergy-focused infant nutrition programs. It benefits from strong regulatory standards and rising consumer preference for organic and clean-label options. Retail pharmacies and hospital channels play a dominant role in distribution. Market players invest in localized marketing and multilingual labeling to enhance regional presence.
Asia Pacific Hypoallergenic Infant Formula For CMPA Market
Asia Pacific holds the highest growth rate in the Hypoallergenic Infant Formula For CMPA Market, with a CAGR of 10.2% and a market share of 38.8% in 2032. The market grows from USD 1,695.8 million in 2024 to USD 3,731.3 million by 2032, driven by rapid urbanization, rising birth rates, and increasing diagnosis of CMPA. China, Japan, India, and South Korea lead demand due to expanding middle-class income and improved pediatric healthcare access. It benefits from rising awareness campaigns and government-backed infant nutrition programs. Online distribution channels grow rapidly in this region. Regional investments in pediatric allergy research further support product innovation.
Latin America Hypoallergenic Infant Formula For CMPA Market
Latin America represents a smaller but steadily growing segment, contributing 2.9% of the global market in 2024. The market is valued at USD 126.4 million in 2024 and projected to reach USD 314.1 million by 2032, at a CAGR of 6.9%. Brazil and Mexico dominate regional sales due to improving healthcare infrastructure and growing infant food sectors. It gains from increasing awareness among healthcare professionals and urban parents. Government programs supporting maternal and child health influence adoption rates. Distribution remains largely reliant on hospitals and retail pharmacies, with online channels showing gradual uptake.
Middle East Hypoallergenic Infant Formula For CMPA Market
The Middle East holds a market value of USD 37.9 million in 2024, expected to rise to USD 123.7 million by 2032, with a CAGR of 8.5%, accounting for about 0.8% of global revenue in 2024. Gulf countries such as the UAE and Saudi Arabia lead market expansion, driven by premium healthcare services and increasing CMPA awareness. It benefits from the growing presence of international formula brands and rising consumer demand for specialized infant nutrition. Retail and pharmacy channels dominate sales distribution. Manufacturers focus on halal-certified and premium-grade formulas to cater to local preferences.
Africa Hypoallergenic Infant Formula For CMPA Market
Africa contributes 0.8% to the global Hypoallergenic Infant Formula For CMPA Market in 2024, with a market size of USD 35.4 million, projected to reach USD 133.3 million by 2032, expanding at a CAGR of 9.3%. South Africa, Nigeria, and Egypt drive market activity through improving maternal health programs and international aid support. It faces constraints from limited purchasing power and uneven product availability. Awareness campaigns by NGOs and partnerships with health ministries aim to improve diagnosis and access. Low-cost and region-specific formulations may unlock future growth. The region holds long-term potential for volume-based expansion.
Key players
- Nestlé Health Science
- Danone Nutricia
- Mead Johnson (Reckitt Benckiser)
- Abbott Laboratories
- FrieslandCampina
- HiPP GmbH & Co
- Hero Group
- Ausnutria
- Meiji Holdings Co., Ltd.
- Perrigo Company plc
- Kabrita (Ausnutria brand)
- Kendamil (Kendal Nutricare)
- Arla Foods Ingredients
- Synutra International, Inc
Competitive Analysis
The Hypoallergenic Infant Formula For CMPA Market is highly competitive and dominated by global nutrition leaders with strong pediatric brand equity and research capabilities. It is shaped by companies like Nestlé Health Science, Danone Nutricia, Abbott, and Mead Johnson, which invest heavily in clinical validation and product innovation. Regional players such as HiPP, Kendamil, and Kabrita enhance diversity with clean-label and goat milk-based formulas. Competitive differentiation stems from ingredient quality, hydrolysis techniques, and distribution reach. Online retail strategies, subscription models, and hospital partnerships strengthen market positioning. Companies focus on expanding footprints in Asia Pacific and Africa to capitalize on high-growth opportunities.
Recent Developments
- In May 2024, Abbott Laboratories released Arize by Similac, a plant-based, hydrolyzed rice protein formula specifically designed for infants with Cow’s Milk Protein Allergy (CMPA). This formula is promoted as 100% plant-based, lactose-free, and clinically hypoallergenic, making it suitable for vegetarian, kosher, and halal diets.
- In March 2023, Danone Nutricia introduced Pepticate, an extensively hydrolyzed powdered formula (eHF) for CMPA, to the U.S. market—importing 50,000 cans (plus Pepticate Syneo) to address formula shortages and provide a trusted, clinically-validated option
Market Concentration and Characteristics
The Hypoallergenic Infant Formula For CMPA Market is moderately concentrated, with a few multinational corporations holding substantial market share through established product lines and global distribution. It features strong brand loyalty, clinical endorsement, and high regulatory compliance, which create barriers for new entrants. Product differentiation centers on protein source, degree of hydrolysis, and formulation purity. The market exhibits high entry costs due to stringent clinical trials and certification requirements. It maintains a premium pricing structure, driven by specialized ingredients and healthcare-driven demand. Innovation, pediatric partnerships, and regional adaptation define key competitive characteristics.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Report Coverage
The research report offers an in-depth analysis based on Product Type, Distribution Channel and Region. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- Global demand will increase as early diagnosis of cow milk protein allergy becomes more common in both developed and emerging healthcare systems.
- Asia Pacific will remain the fastest-growing region due to improving healthcare access, rising birth rates, and greater consumer awareness of infant allergies.
- Future formulations will emphasize organic certification, non-GMO ingredients, and minimal processing to meet rising clean-label demand.
- Amino acid-based formulas will capture a larger share of the market as clinical preference grows for treating severe CMPA cases.
- Digital platforms, teleconsultations, and subscription models will streamline product access and strengthen direct-to-consumer engagement.
- Companies will invest in refining hydrolysis and fermentation technologies to improve efficacy, taste, and nutritional value.
- Emerging regional brands will expand by offering affordable, locally adapted formulas aligned with cultural and dietary preferences.
- Policy initiatives aimed at maternal and infant health will promote wider adoption of hypoallergenic formulas through public health channels.
- Sustainable sourcing, eco-friendly packaging, and carbon-neutral production will become key differentiators in brand selection.
- Strong alliances with pediatricians, allergists, and healthcare institutions will influence product development and help penetrate new markets.





