Market Overview
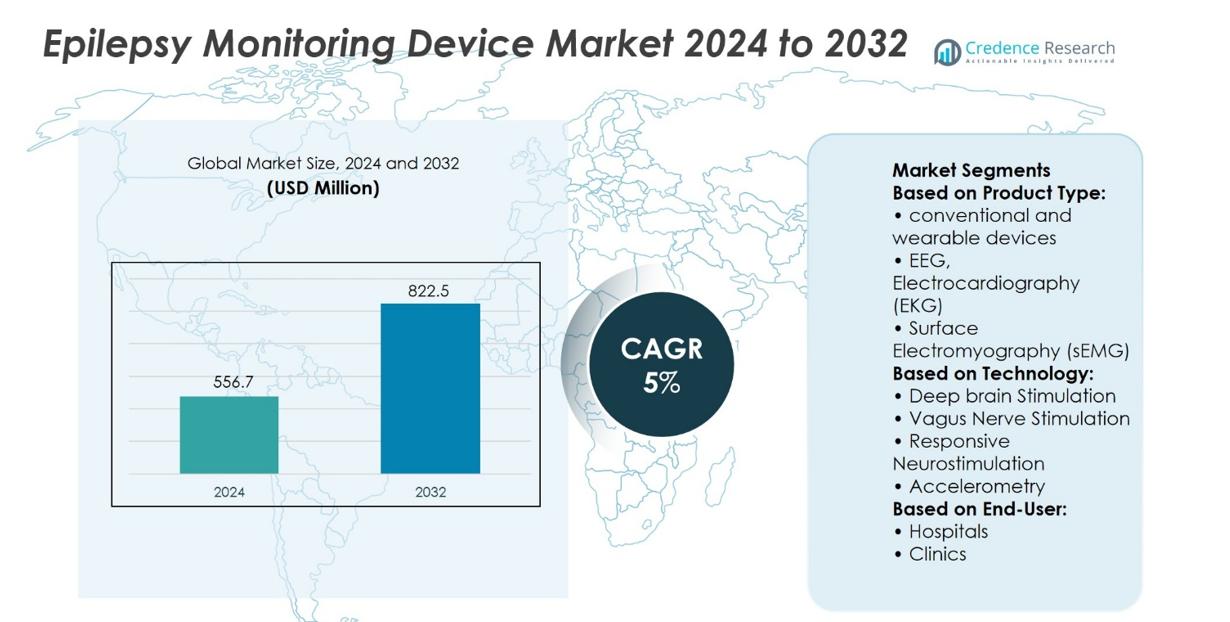
Epilepsy Monitoring Device Market size was valued at USD 556.7 million in 2024 and is anticipated to reach USD 822.5 million by 2032, at a CAGR of 5% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Epilepsy Monitoring Device Market Size 2024 |
USD 556.7 million |
| Epilepsy Monitoring Device Market, CAGR |
5% |
| Epilepsy Monitoring Device Market Size 2032 |
USD 822.5 million |
The Epilepsy Monitoring Device Market grows on strong drivers such as rising prevalence of epilepsy, increasing demand for continuous monitoring, and emphasis on patient safety supported by advanced diagnostic tools. It benefits from technological progress in wearable systems, cloud connectivity, and AI-driven analytics that enhance seizure detection and treatment planning. The market reflects trends toward remote monitoring, integration of multi-parameter platforms, and expansion of home-based solutions that improve accessibility. Healthcare providers adopt data-driven tools to optimize personalized care, while growing investments in digital health and telemedicine accelerate the shift toward smarter, patient-centric monitoring ecosystems.
The Epilepsy Monitoring Device Market shows strong geographical presence, with North America leading due to advanced healthcare infrastructure, followed by Europe with robust neurology networks and Asia-Pacific emerging through expanding medical investments. Latin America and the Middle East & Africa demonstrate steady adoption supported by improving access to specialized care. Key players shaping the market include Stratus, Natus Medical, GE Healthcare, Cadwell Industries, BrainScope, Seer Medical, Medtronic, Compumedics, Koninklijke Philips, and Nihon Kohden, each driving innovation and broader market penetration.
Market Insights
- The Epilepsy Monitoring Device Market size was valued at USD 556.7 million in 2024 and is projected to reach USD 822.5 million by 2032, at a CAGR of 5%.
- Rising prevalence of epilepsy and growing demand for continuous monitoring drive adoption of advanced devices.
- Technological innovation in wearable systems, AI-enabled analytics, and cloud connectivity strengthens precision in seizure detection.
- Competition is shaped by companies focusing on EEG systems, remote monitoring platforms, and integrated multi-parameter solutions.
- High cost of advanced devices and limited access in developing regions act as key restraints.
- North America leads the market, followed by Europe, while Asia-Pacific shows rapid growth through healthcare investments.
- Latin America and the Middle East & Africa demonstrate steady adoption, supported by expanding healthcare infrastructure and awareness initiatives.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers
Rising Global Burden of Epilepsy and Demand for Continuous Monitoring Solutions
The Epilepsy Monitoring Device Market is strongly influenced by the growing prevalence of epilepsy worldwide. Neurological disorders affect millions of patients, creating a consistent need for reliable diagnostic and monitoring solutions. Healthcare providers require precise tools to track seizure patterns and evaluate treatment response effectively. It drives investments into advanced monitoring systems that support both clinical and home-based use. Governments and international health organizations highlight epilepsy as a public health concern, which raises awareness and accelerates adoption of modern devices. This trend establishes a critical foundation for steady market demand.
- For instance, BioSerenity, a Paris-based medtech firm, contributed to the diagnosis of 30 million patients per year through its ambulatory smart clothing platform that integrates medical sensors, a mobile app, and AI-driven data analysis.
Technological Advancements Driving Innovation in Wearable and Remote Devices
The Epilepsy Monitoring Device Market benefits from rapid technological progress in wearable sensors, cloud connectivity, and mobile applications. Companies invest in systems that allow patients to record seizure activity in real time and transmit data directly to physicians. It enhances accuracy and reduces reliance on self-reported events, which often lack precision. AI-powered analytics improve predictive capabilities, enabling earlier detection of seizure episodes. Healthcare systems view remote monitoring as a cost-effective way to manage large patient populations. These innovations push the market toward smarter, more user-friendly solutions.
- For instance, Philips Healthcare operates remote monitoring systems that collectively support monitoring for over 750 million patients globally per year.
Growing Focus on Patient Safety and Personalized Treatment Strategies
The Epilepsy Monitoring Device Market gains momentum from the emphasis on reducing seizure-related risks and tailoring therapy to individual patients. Hospitals and clinics adopt continuous monitoring tools to improve treatment planning and minimize emergency admissions. It allows neurologists to access long-term data, which strengthens decision-making in drug-resistant epilepsy cases. Devices capable of integrating EEG, motion tracking, and biosignal monitoring enhance clinical value. The focus on safety drives demand for portable solutions that protect patients outside hospital settings. Personalized approaches encourage wider adoption across diverse healthcare systems.
Expanding Healthcare Infrastructure and Rising Adoption in Emerging Economies
The Epilepsy Monitoring Device Market grows with the expansion of healthcare infrastructure in emerging regions. Governments allocate funding for neurological care and modern diagnostic systems, creating opportunities for device manufacturers. It strengthens availability of monitoring services in areas previously underserved by specialized care. Private hospitals and research institutions invest in advanced monitoring centers to improve clinical outcomes. Growing access to health insurance in developing economies further supports affordability of such devices. This development ensures stronger long-term adoption across both established and emerging markets.
Market Trends
Integration of Artificial Intelligence and Predictive Analytics into Monitoring Platforms
The Epilepsy Monitoring Device Market reflects a strong trend toward incorporating artificial intelligence into clinical workflows. Developers create platforms that analyze large volumes of EEG and biosignal data to identify subtle seizure indicators. It improves the predictive accuracy of monitoring systems and supports early intervention strategies. Hospitals and research centers deploy AI-driven tools to manage complex epilepsy cases with higher precision. Continuous data analysis allows healthcare providers to optimize treatment while reducing trial-and-error approaches. This shift positions intelligent monitoring platforms as a cornerstone of future epilepsy management.
- For instance, the AI model SCORE‑AI was trained using 30,493 clinical EEG recordings to automatically distinguish normal from abnormal readings and further classify abnormal traces into epileptiform focal patterns.
Rising Adoption of Wearable and Home-Based Monitoring Technologies
The Epilepsy Monitoring Device Market shows increasing demand for compact and wearable systems designed for continuous use. Patients seek solutions that enable discreet monitoring while maintaining mobility and independence. It encourages companies to introduce lightweight devices with wireless connectivity for real-time data transfer. Physicians gain access to consistent data outside hospital settings, which strengthens diagnostic accuracy. The focus on patient comfort drives acceptance of consumer-friendly designs. This trend underscores the importance of user-centered innovation in expanding market reach.
- For instance, in a remote heart‑rate variability biofeedback study using wearable devices, over 51 million data points were collected from participants demonstrating the high scale and real-world efficacy of wearable health monitoring technologies.
Expansion of Telemedicine and Remote Monitoring Capabilities Across Healthcare Systems
The Epilepsy Monitoring Device Market benefits from the global acceleration of telemedicine adoption. Remote patient management platforms integrate monitoring devices with secure cloud systems for seamless physician access. It enables neurologists to evaluate seizure activity without requiring frequent hospital visits. The approach reduces costs for healthcare providers and increases convenience for patients. Hospitals leverage remote monitoring networks to manage large patient bases across urban and rural regions. This trend ensures broader availability of specialized neurological care.
Increasing Emphasis on Multi-Parameter Monitoring and Data Integration for Personalized Care
The Epilepsy Monitoring Device Market demonstrates a shift toward devices capable of capturing multiple physiological signals. Manufacturers design systems that integrate EEG, heart rate, oxygen saturation, and motion analysis into a single platform. It allows physicians to assess seizure activity within a broader clinical context. Multi-parameter data improves accuracy in identifying seizure triggers and patterns. Healthcare professionals use integrated platforms to design personalized treatment plans that extend beyond seizure control. This trend highlights the importance of comprehensive monitoring solutions in modern epilepsy care.
Market Challenges Analysis
High Cost of Advanced Devices and Limited Accessibility Across Developing Regions
The Epilepsy Monitoring Device Market faces a major challenge due to the high cost of advanced monitoring systems. Sophisticated EEG-based and wearable solutions require significant investment, which limits adoption in low- and middle-income countries. It creates disparities in access, with patients in developed markets benefiting more from cutting-edge devices. Hospitals in emerging regions often lack infrastructure to support long-term monitoring programs. Insurance coverage gaps further restrict affordability for patients who require continuous care. The issue of cost and accessibility slows down global expansion of advanced monitoring technologies.
Technical Complexities, Data Management Issues, and Regulatory Barriers
The Epilepsy Monitoring Device Market encounters obstacles linked to technical and operational complexities. Devices generate millions of data points that require secure storage, efficient processing, and accurate interpretation. It places pressure on healthcare providers to invest in robust IT infrastructure and skilled personnel. Concerns over patient data privacy and cybersecurity remain significant, especially with cloud-based systems. Strict regulatory requirements delay product approvals and extend time-to-market for new technologies. These challenges demand coordinated strategies from manufacturers, regulators, and healthcare institutions to ensure wider adoption.
Market Opportunities
Expanding Role of Digital Health Integration and AI-Enabled Monitoring Platforms
The Epilepsy Monitoring Device Market presents strong opportunities through integration with digital health ecosystems. AI-powered platforms provide real-time seizure prediction and advanced analytics that enhance clinical decision-making. It enables healthcare professionals to design personalized treatment pathways supported by continuous data insights. Cloud connectivity and mobile applications extend monitoring capabilities beyond hospital walls, increasing patient engagement and physician oversight. The combination of predictive analytics and remote access positions AI-enabled solutions as critical tools for the next phase of epilepsy care. This creates room for innovation that improves both outcomes and patient safety.
Rising Demand in Emerging Economies and Growth of Home-Based Monitoring Solutions
The Epilepsy Monitoring Device Market holds opportunities in developing regions where healthcare infrastructure is expanding rapidly. Governments allocate funding for neurological care programs, creating demand for modern monitoring technologies. It encourages manufacturers to design cost-effective devices suited to large patient populations. Home-based monitoring systems gain acceptance among patients seeking comfort, independence, and consistent tracking of seizure activity. Growing awareness of epilepsy as a public health priority strengthens adoption in markets previously underserved by advanced diagnostics. This development widens the pathway for long-term global growth of epilepsy monitoring technologies.
Market Segmentation Analysis:
By Product Type
The Epilepsy Monitoring Device Market is segmented by product type into conventional and wearable devices, EEG, electrocardiography (EKG), surface electromyography (sEMG), video detection systems, and others. EEG devices hold a significant role since they provide detailed brain wave data that remains central to diagnosis and long-term seizure tracking. Wearable devices gain attention due to their portability and patient-friendly design, supporting continuous monitoring outside hospital settings. It expands adoption among patients who require real-time data without constant clinical supervision. Video detection systems and sEMG devices complement EEG by adding motion or muscle activity records, offering multi-parameter perspectives for clinicians. The segment demonstrates a steady transition from conventional systems to smart, connected solutions.
- For instance, BioSerenity, a Paris-based medtech firm, employs its Neuronaute® wearable EEG and smart-clothing system to facilitate diagnosis for approximately 30,000 patients per year, reflecting the real-world scale and adoption of wearable EEG technology in epilepsy care.
By Technology
The Epilepsy Monitoring Device Market is also segmented by technology into deep brain stimulation, vagus nerve stimulation, responsive neurostimulation, and accelerometry. Deep brain stimulation systems are widely deployed for patients with drug-resistant epilepsy, offering targeted neuromodulation that reduces seizure frequency. Vagus nerve stimulation is adopted for long-term therapy, supported by implantable devices that provide consistent electrical stimulation. It strengthens treatment options where conventional monitoring alone is insufficient. Responsive neurostimulation gains traction as it combines detection with immediate intervention, enabling a more proactive approach. Accelerometry-based systems serve as supportive tools for motion detection, enhancing wearable device capabilities and alert mechanisms. Each technology demonstrates unique clinical value in broadening patient care strategies.
- For instance, LivaNova, a key player in vagus nerve stimulation (VNS), reported that its VNS Therapy devices have been used in treatment for more than 1.2 million patients globally across epilepsy and depression indications.
By End User
The Epilepsy Monitoring Device Market is segmented by end user into hospitals and clinics. Hospitals dominate due to their specialized neurology departments, advanced diagnostic infrastructure, and ability to integrate multi-parameter monitoring systems. It positions them as primary hubs for both acute and long-term epilepsy care. Clinics play an increasing role by offering accessible and cost-effective monitoring services to outpatients. The demand for portable and wearable solutions supports adoption in smaller healthcare facilities where patients seek convenience. Both hospitals and clinics adopt devices that enhance efficiency, improve diagnostic accuracy, and support continuity of care. This segment highlights the balance between advanced institutional infrastructure and growing community-based care delivery.
Segments:
Based on Product Type:
- Conventional and wearable devices
- EEG, Electrocardiography (EKG)
- Surface Electromyography (sEMG)
Based on Technology:
- Deep brain Stimulation
- Vagus Nerve Stimulation
- Responsive Neurostimulation
- Accelerometry
Based on End-User:
Based on the Geography:
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis
North America
North America accounts for the largest share of the Epilepsy Monitoring Device Market, contributing 38% of the global share. The region benefits from advanced healthcare infrastructure, high awareness of neurological disorders, and widespread adoption of digital monitoring technologies. Hospitals and specialized clinics invest heavily in EEG systems, responsive neurostimulation, and wearable devices, ensuring comprehensive coverage for patients. It also reflects strong regulatory support, with the U.S. Food and Drug Administration (FDA) approving multiple advanced monitoring platforms in recent years. Insurance coverage for epilepsy management further drives patient access to modern diagnostic and treatment options. The concentration of leading manufacturers and ongoing clinical research reinforces North America’s leading position in this market.
Europe
Europe represents 27% of the Epilepsy Monitoring Device Market, supported by a combination of robust healthcare systems and high prevalence of epilepsy across major countries such as Germany, the United Kingdom, France, and Italy. Hospitals in the region deploy advanced EEG and video detection systems as standard diagnostic practice, while wearable monitoring devices gain traction in outpatient and home-based care. It benefits from a strong regulatory framework under the European Medicines Agency and active funding for neurological research programs. Governments promote adoption of monitoring systems as part of initiatives to improve chronic disease management and reduce hospitalization rates. The region’s emphasis on patient-centered healthcare and integration of digital monitoring platforms contributes to sustained growth.
Asia-Pacific
Asia-Pacific accounts for 22% of the Epilepsy Monitoring Device Market and demonstrates strong growth potential due to its expanding healthcare infrastructure and rising awareness of neurological disorders. Countries such as China, India, Japan, and South Korea increase investment in advanced medical devices, including EEG and wearable monitoring systems. It reflects higher patient demand driven by large population bases and rising incidence of epilepsy. Governments allocate funding for modern diagnostic centers, while private hospitals adopt innovative devices to improve treatment outcomes. Partnerships between global manufacturers and local distributors enhance product availability across the region. Asia-Pacific continues to evolve into a priority market for long-term expansion of epilepsy monitoring technologies.
Latin America
Latin America contributes 7% of the Epilepsy Monitoring Device Market, with adoption concentrated in countries such as Brazil, Mexico, and Argentina. Hospitals in urban centers invest in EEG devices and surface electromyography systems to support accurate diagnosis and monitoring. It demonstrates a gradual shift toward wearable and home-based monitoring solutions, although cost remains a significant barrier to widespread adoption. Governments and non-governmental organizations run awareness programs to reduce treatment gaps for epilepsy patients. Growth is further supported by expanding private healthcare networks that invest in advanced monitoring infrastructure. Latin America’s steady progress highlights opportunities for broader market penetration over the coming decade.
Middle East and Africa
The Middle East and Africa account for 6% of the Epilepsy Monitoring Device Market, representing the smallest share but showing steady improvement. The region faces challenges such as limited specialized neurological facilities and lower affordability of advanced devices. It reflects increasing adoption in Gulf countries like Saudi Arabia and the United Arab Emirates, where hospitals integrate EEG and video detection systems into specialized care programs. Africa demonstrates slower adoption due to resource constraints, although international aid and collaborative health projects enhance access to diagnostic solutions. Growing investments in healthcare modernization and government-backed initiatives create opportunities for gradual expansion. The market in this region continues to strengthen with increasing awareness and infrastructure development.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Stratus
- Natus Medical
- GE Healthcare
- Cadwell Industries, Inc.
- BrainScope Company, Inc.
- Seer Medical
- Medtronic
- Compumedics Limited
- Koninklijke Philips N.V.
- NIHON KOHDEN CORPORATION
Competitive Analysis
The leading players in the Epilepsy Monitoring Device Market include Stratus, Natus Medical, GE Healthcare, Cadwell Industries, Inc., BrainScope Company, Inc., Seer Medical, Medtronic, Compumedics Limited, Koninklijke Philips N.V., and NIHON KOHDEN CORPORATION. The Epilepsy Monitoring Device Market is highly competitive, shaped by a mix of established medical device manufacturers and emerging innovators specializing in neurological care. Companies focus on expanding product portfolios with advanced EEG systems, wearable solutions, and remote monitoring platforms that enhance both hospital-based and home-based care. Strategic investments in artificial intelligence, cloud integration, and predictive analytics strengthen the accuracy of seizure detection and long-term patient management. The market reflects continuous efforts to secure regulatory approvals and expand global distribution networks to reach underserved regions. Competition also centers on partnerships with hospitals, research institutions, and telemedicine providers to integrate monitoring devices into comprehensive epilepsy management programs. The landscape emphasizes innovation, accessibility, and improved patient outcomes as primary drivers of competitive positioning.
Recent Developments
- In April 2025, Epiminder received FDA de novo clearance for the Minder implantable EEG system, enabling months of continuous brain monitoring in the United States.
- In June 2024, researchers from the University of Southern California (USC) developed a new artificial intelligence (AI) system to accurately detect/identify epileptic seizures, including rare and complex cases, even in young children.
- In February 2024, Empatica Inc., a pioneering company in continuous, noninvasive, and discreet monitoring for neurological conditions, announced the launch of EpiMonitor.
- In October 2023, NeuroPace, a commercial-stage medical device company, announced enhancements to its RNS System for epilepsy management, including the nSight Platform, Simple Set Programming, and Tablet Remote Monitor.
Market Concentration & Characteristics
The Epilepsy Monitoring Device Market reflects moderate concentration, with a blend of global medical device corporations and specialized neurodiagnostic companies competing for share. It is characterized by continuous innovation in EEG systems, wearable monitoring platforms, and digital integration that supports both hospital and home-based care. The market shows strong differentiation through advanced features such as multi-parameter tracking, predictive analytics, and AI-enabled diagnostic support. Regulatory compliance and clinical validation remain critical factors that shape competitive positioning, while high development costs create barriers for new entrants. It emphasizes patient safety, long-term data accuracy, and seamless connectivity with clinical workflows. The structure highlights a balance between established players leveraging scale and emerging firms introducing disruptive technologies.
Report Coverage
The research report offers an in-depth analysis based on Product Type, Technology, End-User and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market will see stronger adoption of AI-driven platforms for early seizure prediction and automated diagnostics.
- Wearable devices will expand their role in home-based epilepsy care with greater patient acceptance.
- Remote monitoring solutions will integrate more deeply with telemedicine platforms for continuous physician oversight.
- Multi-parameter systems will gain preference as they combine EEG, motion, and cardiovascular tracking in one platform.
- Regulatory support will increase for digital health solutions, accelerating product approvals and market entry.
- Emerging economies will invest in advanced neurological care, creating new growth opportunities.
- Cloud-based data management will become standard for long-term storage and secure sharing of patient information.
- Personalized treatment approaches will rely on continuous monitoring to optimize therapy effectiveness.
- Collaborations between hospitals, research centers, and technology providers will expand innovation pipelines.
- The market will continue to shift toward patient-centric designs that emphasize comfort, accuracy, and accessibility.




