Market Overview:
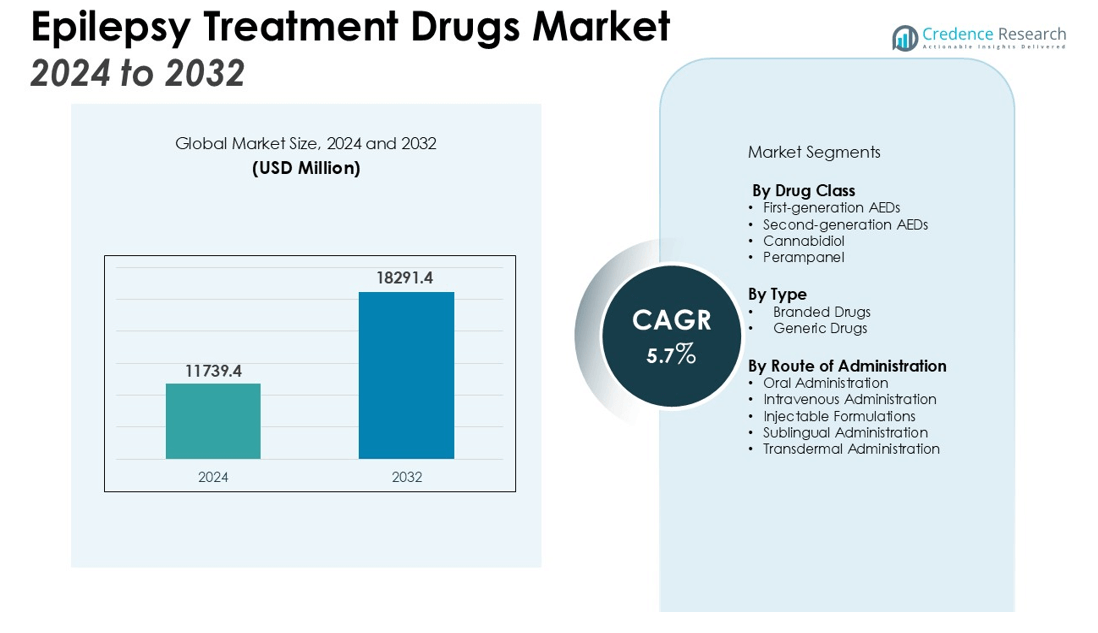
The Epilepsy Treatment Drugs Market size was valued at USD 11739.4 million in 2024 and is anticipated to reach USD 18291.4 million by 2032, at a CAGR of 5.7% during the forecast period (2024-2032).
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2019-2022 |
| Base Year |
2023 |
| Forecast Period |
2024-2032 |
| Epilepsy Treatment Drugs Market Size 2024 |
USD 11739.4 million |
| Epilepsy Treatment Drugs Market, CAGR |
5.7% |
| Epilepsy Treatment Drugs Market Size 2032 |
USD 18291.4 million |
Key market drivers include the rising prevalence of epilepsy, particularly in developing regions, where healthcare infrastructure is improving. Moreover, the continuous development of antiepileptic drugs (AEDs), including novel drug classes such as perampanel and cannabidiol, is enhancing treatment options for patients, improving outcomes, and reducing side effects. Increasing healthcare expenditures and a greater push for early diagnosis and treatment are also contributing to market growth. The growing demand for precision medicine and the expanding use of advanced diagnostics also play a crucial role in shaping the market dynamics.
Regionally, North America holds the largest share in the epilepsy treatment drugs market, driven by the high availability of advanced healthcare systems and drug development. Europe follows closely, with a significant demand for AEDs across countries with well-established healthcare infrastructures. The Asia-Pacific region is expected to exhibit the highest growth rate during the forecast period, due to the increasing number of epilepsy cases and improving healthcare accessibility. Additionally, the growing adoption of generic AEDs in emerging markets is likely to accelerate the market’s expansion in these regions.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights:
- The Epilepsy Treatment Drugs Market was valued at USD 11,739.4 million in 2024 and is projected to reach USD 18,291.4 million by 2032, growing at a CAGR of 5.7% from 2024 to 2032.
- Rising global prevalence of epilepsy, particularly in developing regions, and improving healthcare access are major drivers for the market’s growth.
- Advancements in AEDs, including novel treatments like perampanel and cannabidiol, are expanding options for patients and improving treatment outcomes.
- The push for early diagnosis and treatment, supported by public awareness and better diagnostic tools, is driving increased demand for AEDs.
- High drug costs and limited insurance coverage, especially in low-income regions, create barriers to accessing AEDs, impacting treatment adherence.
- North America holds the largest market share, accounting for 40% of the global market, supported by advanced healthcare systems and innovative AED treatments.
- The Asia-Pacific region is expected to grow the fastest, driven by increasing healthcare access, urbanization, and a focus on neurological disorder treatment in countries like China and India.
Market Drivers:
Rising Prevalence of Epilepsy and Growing Healthcare Access
The increasing global prevalence of epilepsy is a major driver for the growth of the Epilepsy Treatment Drugs Market. Epilepsy affects over 50 million people worldwide, with a significant proportion of cases emerging in low- and middle-income countries. Improving healthcare access in these regions is critical for enhancing treatment availability. This growing demand for effective treatment solutions is propelling market expansion, as more patients seek better management of the condition.
- For instance, a large, pooled analysis from the real-world EXPERIENCE study assessed the effectiveness of UCB’s BRIVIACT® (brivaracetam) by including 1,644 adult patients, demonstrating a substantial commitment to evaluating new AEDs in real-world clinical settings.
Advancements in Antiepileptic Drug Development
Innovations in antiepileptic drugs (AEDs) significantly influence the growth of the Epilepsy Treatment Drugs Market. New drug classes such as perampanel and cannabidiol offer improved efficacy and reduced side effects compared to older treatments. The continuous development of AEDs allows for more tailored therapies, addressing the specific needs of patients with various forms of epilepsy. These advancements are improving patient outcomes and broadening treatment options, driving market demand.
Increased Focus on Early Diagnosis and Treatment
The push for earlier diagnosis and treatment of epilepsy is boosting the Epilepsy Treatment Drugs Market. Early detection improves the chances of effective management, reducing the risk of long-term complications. Increased public awareness and advancements in diagnostic tools allow for quicker identification of epilepsy cases. As healthcare systems invest more in preventive care, the demand for effective epilepsy drugs continues to rise, fueling market growth.
- For instance, the Penn State Neuroscience Institute utilizes dense-array EEG, a noninvasive technology that records brain activity with up to 256 electrodes, a significant increase over the 19-21 electrodes in standard EEGs for more precise seizure localization.
Government Initiatives and Healthcare Investments
Government initiatives and increasing healthcare investments are accelerating the growth of the Epilepsy Treatment Drugs Market. In many countries, the focus is shifting towards enhancing healthcare infrastructure and funding research into neurological disorders like epilepsy. The introduction of national healthcare programs that cover epilepsy treatments is promoting market expansion. With continued investment in both research and healthcare services, the availability of treatments is expected to increase, driving further market growth.
Market Trends:
Shift Toward Personalized Medicine in Epilepsy Treatment
A key trend in the Epilepsy Treatment Drugs Market is the increasing adoption of personalized medicine. Tailored treatments based on genetic and molecular profiles of patients are improving the effectiveness of therapies. The focus is shifting from one-size-fits-all approaches to more individualized regimens that consider the specific needs of each patient. Advances in genetic testing and biomarker discovery are enabling healthcare providers to better understand how different patients respond to various antiepileptic drugs (AEDs). This trend is expected to enhance treatment outcomes and reduce adverse effects, making epilepsy management more efficient and targeted. Personalized medicine is gaining traction as healthcare systems recognize the potential for improved quality of life for epilepsy patients through customized therapies.
- For instance, in a study published in JAMA Neurology, Invitae found that for patients with epilepsy, clinical management was changed for 208 out of 418 patients (49.8%) based on genetic diagnoses, with 108 patients experiencing seizure reduction or elimination after treatment changes.
Growing Popularity of Non-Traditional and Plant-Based Therapies
The increasing acceptance and demand for non-traditional treatments in the Epilepsy Treatment Drugs Market reflect another notable trend. Cannabidiol (CBD) and other plant-based therapies are gaining traction as effective alternatives for patients who do not respond well to conventional AEDs. Clinical studies supporting the efficacy of CBD for seizure management are influencing its broader use in epilepsy treatment. Patients and healthcare providers are turning to these alternative therapies as adjunct treatments or in cases where standard AEDs are not sufficiently effective. The rise of these non-pharmacological options is reshaping the market landscape, broadening treatment choices and catering to patients seeking natural or less invasive treatment methods.
- For instance, in an open-label study of patients with treatment-resistant, childhood-onset epilepsy, treatment with a cannabidiol (CBD) oral solution reduced the median monthly frequency of motor seizures from 30.0 at baseline to 15.8 during the 12-week treatment period.
Market Challenges Analysis:
High Costs and Affordability Issues
One of the significant challenges facing the Epilepsy Treatment Drugs Market is the high cost of antiepileptic drugs (AEDs). While advancements in drug development offer more effective treatment options, these drugs often come with steep price tags, limiting access for patients in low-income regions. High drug prices, coupled with limited insurance coverage in certain areas, hinder the affordability of epilepsy treatment. This creates a barrier for many patients who struggle to access necessary medications, impacting overall treatment adherence and health outcomes. Addressing these affordability concerns is essential to ensuring that effective therapies reach a broader patient population.
Side Effects and Treatment Adherence Concerns
Another challenge in the Epilepsy Treatment Drugs Market is the issue of side effects associated with many epilepsy drugs. While AEDs are effective in controlling seizures, they often come with adverse effects such as dizziness, fatigue, and cognitive impairment. These side effects can lead to poor patient adherence to prescribed regimens, particularly in long-term treatment plans. Inconsistent medication usage due to side effects complicates epilepsy management, affecting both the patients’ quality of life and the effectiveness of the drugs. Ensuring the development of drugs with fewer side effects will be crucial for improving patient compliance and treatment outcomes.
Market Opportunities:
Expansion of Generic Epilepsy Medications
One of the key opportunities in the Epilepsy Treatment Drugs Market lies in the growing demand for generic antiepileptic drugs (AEDs). As patents for several branded AEDs expire, generic versions are entering the market, offering more affordable alternatives for patients. This shift enables greater access to treatment, particularly in low- and middle-income regions where cost is a significant barrier. The availability of generics can also increase market competition, driving down prices and improving accessibility. This presents a significant opportunity for both established pharmaceutical companies and new entrants to cater to a broader patient base while maintaining profitability through increased volume.
Advancements in Epilepsy Drug Development
The ongoing advancements in epilepsy drug development present another promising opportunity for the Epilepsy Treatment Drugs Market. Research into novel drug classes, such as cannabinoid-based therapies and precision medicine, opens the door to more effective and personalized treatment options. With the rise in understanding of epilepsy’s genetic and molecular underpinnings, there is potential for new drugs tailored to specific subtypes of epilepsy. This trend not only enhances treatment efficacy but also reduces side effects, improving patient compliance and outcomes. Pharmaceutical companies investing in these areas stand to benefit from unmet medical needs, positioning themselves as leaders in the evolving market.
Market Segmentation Analysis:
By Drug Class
The Epilepsy Treatment Drugs Market includes various drug classes, such as first-generation AEDs, second-generation AEDs, and newer treatments like cannabidiol and perampanel. Second-generation AEDs are increasingly favored due to their improved safety profile and fewer side effects compared to first-generation drugs. These advancements cater to a broader patient base, including those with refractory epilepsy, contributing to the growing adoption of second-generation AEDs.
By Type
The market is segmented into branded and generic drugs. Generic drugs are experiencing rapid growth due to their cost-effectiveness and accessibility, making them a preferred option in emerging markets. While branded drugs still hold a significant market share, the demand for newer, more effective treatments with fewer adverse effects supports the continued relevance of branded AEDs.
- For instance, in a Phase III study for its drug Afinitor®, Novartis showed the treatment significantly reduced treatment-resistant seizures associated with tuberous sclerosis complex (TSC) when used with other anti-epileptic drugs.
By Route of Administration
Oral administration dominates the market, with most AEDs available in tablet or liquid form. Intravenous administration is also critical, particularly for patients requiring rapid seizure control. Injectable formulations are seeing increased demand, driven by the need for emergency treatment in hospitals. Emerging routes like sublingual and transdermal are gaining attention but remain a smaller market segment.
- For instance, UCB’s Vimpat (lacosamide) injection is administered intravenously over a period of 30 to 60 minutes, allowing for controlled delivery in a hospital setting.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Segmentations:
By Drug Class
- First-generation AEDs
- Second-generation AEDs
- Cannabidiol
- Perampanel
By Type
- Branded Drugs
- Generic Drugs
By Route of Administration
- Oral Administration
- Intravenous Administration
- Injectable Formulations
- Sublingual Administration
- Transdermal Administration
By Region
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis:
North America: Market Leader in Epilepsy Treatment Drugs
North America holds the largest share of the Epilepsy Treatment Drugs Market, accounting for 40% of the global market in 2024. The region’s dominance is driven by well-established healthcare systems, high healthcare expenditures, and a strong pipeline of advanced treatments. The United States, in particular, has a large patient population with access to innovative therapies, including both new-generation antiepileptic drugs (AEDs) and personalized treatment options. The region also benefits from extensive research and development efforts by leading pharmaceutical companies, contributing to continuous improvements in epilepsy management. The increasing adoption of generics and favorable reimbursement policies further support the growth of the market in this region.
Europe: Steady Demand and Growing Research Initiatives
Europe accounts for 30% of the Epilepsy Treatment Drugs Market share and demonstrates steady demand for AEDs across several countries. Established healthcare infrastructure and a strong focus on research contribute to the region’s growth in the epilepsy treatment sector. Countries like Germany, the UK, and France are key markets for epilepsy drugs, supported by well-funded healthcare systems and access to cutting-edge treatments. Europe also benefits from a growing emphasis on early diagnosis and better access to treatment, which contributes to increasing patient adherence to prescribed therapies. The rising number of epilepsy cases in Eastern Europe is expected to drive future growth in the region.
Asia-Pacific: Rapid Growth and Increasing Healthcare Accessibility
The Asia-Pacific region holds a 20% share of the Epilepsy Treatment Drugs Market and is expected to witness the highest growth rate during the forecast period. Rapid urbanization, improving healthcare infrastructure, and an increasing focus on neurological disorders are driving market expansion. Countries such as China, India, and Japan are witnessing a rise in epilepsy cases, coupled with improving access to quality healthcare services. The region’s growing middle class and government initiatives to expand healthcare coverage play a pivotal role in increasing the availability of epilepsy treatments. The market is expected to experience accelerated growth due to rising awareness and the availability of more affordable treatment options in emerging economies.
Key Player Analysis:
- Neurelis
- Novartis
- AbbVie
- Bausch Health Companies
- Dr. Reddy’s Laboratories
- Eisai
- Pfizer
- Sanofi
- GSK
- SK Biopharmaceuticals
- Jazz Pharmaceuticals
- Lupin Pharmaceuticals
Competitive Analysis:
The Epilepsy Treatment Drugs Market is highly competitive, with several key players driving innovation and market expansion. Leading pharmaceutical companies such as UCB, Eisai, and GlaxoSmithKline dominate the market, offering a range of antiepileptic drugs (AEDs) that cater to different types of epilepsy. These companies invest heavily in research and development to introduce novel drugs with improved efficacy and safety profiles. The market is also witnessing an increasing presence of generic drug manufacturers, such as Teva Pharmaceuticals and Mylan, providing cost-effective alternatives to branded drugs, particularly in emerging markets. Strategic collaborations, mergers, and acquisitions are common as companies seek to expand their product portfolios and strengthen their market position. The growing focus on precision medicine and personalized treatments also intensifies competition, as firms aim to develop tailored therapies that meet the specific needs of patients with epilepsy.
Recent Developments:
- In May 2025, AbbVie announced a collaboration and license option agreement with ADARx Pharmaceuticals to develop next-generation siRNA therapies.
- In July 2025, Bausch Health announced its plan to redeem $602 million of its 9.25% Senior Notes due in 2026 as part of its debt reduction initiatives.
Market Concentration & Characteristics:
The Epilepsy Treatment Drugs Market exhibits a moderate to high concentration, with a few key players controlling a significant share of the market. Leading pharmaceutical companies like UCB, Eisai, and GlaxoSmithKline dominate the market through their extensive portfolios of branded AEDs. These companies benefit from strong brand recognition, advanced R&D capabilities, and established distribution networks. The market also sees growing competition from generic drug manufacturers, such as Teva Pharmaceuticals, who offer affordable alternatives, increasing market accessibility, particularly in low- and middle-income regions. The entry of smaller biotech firms with novel treatments adds diversity to the competitive landscape, focusing on improving efficacy and minimizing side effects. Regulatory approval processes and patent protections remain crucial factors that influence market concentration, as companies strive to maintain exclusive market positions and protect their innovations.
Report Coverage:
The research report offers an in-depth analysis based on Drug Class, Type, Route of Administration and Region. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook:
- The demand for personalized medicine will rise as advancements in genetics and biomarkers allow for more tailored epilepsy treatments.
- Novel antiepileptic drugs (AEDs) targeting specific genetic markers are expected to play a significant role in improving treatment outcomes.
- Generic AEDs will continue to dominate, especially in emerging markets, due to their affordability and increased availability.
- The increasing focus on early diagnosis and treatment will drive the demand for AEDs, promoting better patient outcomes and reducing long-term complications.
- Innovative drug delivery systems, including sublingual and transdermal options, will gain traction, offering patients more convenient and effective treatment choices.
- The expansion of healthcare access in developing regions, coupled with government initiatives, will lead to greater adoption of epilepsy treatment drugs.
- The market will see a growing number of collaborations and partnerships between pharmaceutical companies and research institutions to accelerate drug development.
- Advances in neuromodulation and surgical treatment options for drug-resistant epilepsy could complement pharmaceutical treatments, diversifying the market.
- The growing awareness of epilepsy, especially in underserved regions, will drive market growth by increasing demand for accessible treatment solutions.
- Increasing investment in research and development will lead to the introduction of new AEDs with better efficacy profiles and fewer side effects, enhancing patient adherence.




