Market Overview:
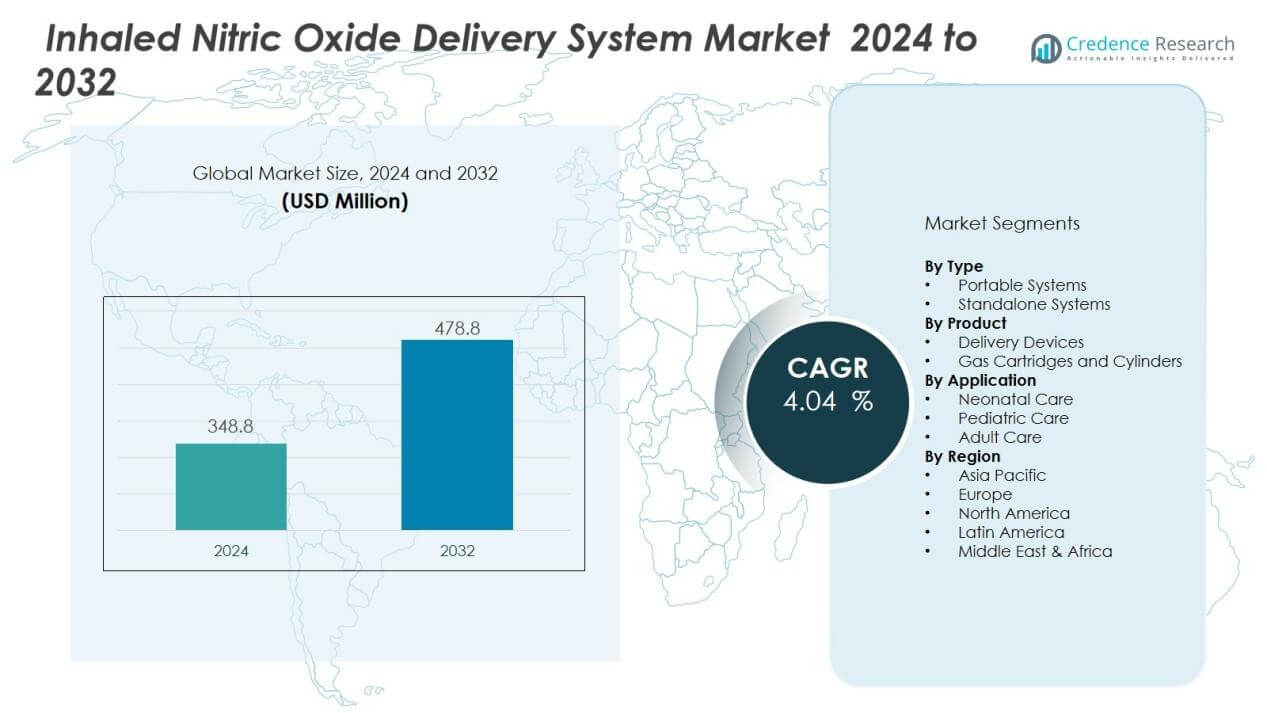
The inhaled nitric oxide delivery system market size was valued at USD 348.8 million in 2024 and is anticipated to reach USD 478.8 million by 2032, at a CAGR of 4.04 % during the forecast period (2024-2032).
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Inhaled Nitric Oxide Delivery Systems Market Size 2024 |
USD 348.8 Million |
| Inhaled Nitric Oxide Delivery Systems Market, CAGR |
4.04 % |
| Inhaled Nitric Oxide Delivery Systems Market Size 2032 |
USD 478.8 Million |
Key drivers include the growing prevalence of pulmonary hypertension, neonatal hypoxic respiratory failure, and acute respiratory distress syndrome (ARDS). The market benefits from the increasing need for targeted therapies that improve oxygenation without systemic side effects. Technological advancements in portable and user-friendly delivery devices, coupled with strong demand in critical care settings, further support growth. Favorable clinical outcomes and rising awareness among healthcare professionals also strengthen adoption rates.
Regionally, North America leads the market due to advanced healthcare infrastructure, high diagnosis rates, and strong presence of key players. Europe follows with robust regulatory frameworks and growing use in neonatal intensive care units. The Asia-Pacific region is expected to record the fastest growth, driven by large patient populations, rising healthcare investments, and expanding neonatal care facilities in countries like India and China. Latin America and the Middle East & Africa also present emerging opportunities as healthcare modernization progresses.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights:
- The inhaled nitric oxide delivery system market was valued at USD 348.8 million in 2024 and is projected to reach USD 478.8 million by 2032.
- Rising prevalence of pulmonary hypertension, neonatal hypoxic respiratory failure, and ARDS continues to drive demand.
- Strong adoption in neonatal intensive care units highlights its role in reducing invasive procedures for infants.
- Technological advancements in portable, user-friendly, and precise delivery devices support wider clinical adoption.
- High treatment costs and limited reimbursement policies remain challenges in emerging markets.
- North America led with 42% share in 2024, supported by advanced infrastructure and strong adoption rates.
- Asia-Pacific, with 19% share in 2024, is expected to grow fastest due to healthcare investments and large neonatal populations.
Market Drivers:
Rising Prevalence of Pulmonary Hypertension and Respiratory Disorders:
The inhaled nitric oxide delivery system market benefits from the increasing incidence of pulmonary hypertension and related respiratory conditions. These systems provide targeted vasodilation, improving oxygenation in critically ill patients. Growing cases of neonatal hypoxic respiratory failure and acute respiratory distress syndrome (ARDS) further fuel demand. It strengthens its role as an essential therapy in intensive care units worldwide.
- For instance, Mallinckrodt’s INOmax EVOLVE DS system utilizes 1.43-lb, 0.4-L mini-cylinders filled to 3000 psi, enabling precise delivery of inhaled nitric oxide to patients in neonatal intensive care units.
Expanding Use in Neonatal and Pediatric Care Settings:
Demand for inhaled nitric oxide delivery systems is driven by their widespread adoption in neonatal intensive care units. The therapy reduces the need for invasive procedures, making it a preferred solution for vulnerable infants. Pediatric applications are also expanding, with growing emphasis on improving survival rates and reducing long-term complications. It creates strong opportunities for specialized devices and tailored treatment protocols.
- For instance, the Beyond Air LungFit™ PH system received FDA approval for term and near-term neonates with hypoxic respiratory failure in 2022 and provides a portable nitric oxide solution that can deliver up to 80 ppm continuously, enhancing safety by allowing on-demand production without storing compressed gas.
Advancements in Portable and User-Friendly Delivery Devices:
Continuous innovation in design and technology drives market growth by improving usability and safety. Portable systems allow flexibility in patient care across hospital departments, emergency settings, and transport situations. Enhanced monitoring features and integration with critical care equipment improve precision in dosing. It ensures broader acceptance among healthcare providers seeking efficiency and reliability.
Growing Healthcare Investments and Awareness Among Professionals:
Rising healthcare expenditures and expansion of advanced care facilities in emerging economies contribute significantly to demand. Awareness programs and training initiatives improve adoption of inhaled nitric oxide therapy among clinicians. Governments and institutions are increasingly supporting access to advanced respiratory treatments in underserved regions. It strengthens the long-term growth outlook for the inhaled nitric oxide delivery system market.
Market Trends:
Integration of Advanced Technologies and Portable Solutions:
The inhaled nitric oxide delivery system market is witnessing strong momentum through the integration of advanced technologies and portable solutions. Manufacturers are focusing on compact, lightweight devices that allow seamless use in critical care, transport, and home care settings. Smart monitoring features, precise dosage control, and compatibility with ventilators improve clinical outcomes and safety. It supports wider adoption across neonatal, pediatric, and adult patient populations. Increasing preference for portable devices reflects the growing demand for flexible and patient-centered care models. Continuous R&D efforts highlight the shift toward innovation and performance-driven designs.
- For Instance, The GeNOsyl DS by Vero Biotech features a backup battery that provides up to one hour of power, facilitating continued therapy during brief periods without a main power source, such as during inter-hospital transport. The device is a portable, tankless system designed for use in acute care settings and relies on a constant AC power supply for extended operation.
Expanding Applications Beyond Traditional Critical Care Use:
Growing adoption of inhaled nitric oxide delivery systems in therapeutic areas beyond traditional neonatal and ARDS care marks a key trend. The therapy is being explored for use in managing conditions such as chronic obstructive pulmonary disease (COPD), pulmonary embolism, and cardiac surgery support. It reflects the increasing confidence in nitric oxide’s targeted mechanism and safety profile. Healthcare providers are broadening treatment protocols to include expanded clinical applications that meet evolving patient needs. Rising global investments in clinical trials and evidence-based research drive this expansion. The inhaled nitric oxide delivery system market gains resilience from its widening role in diverse healthcare settings.
- For Instance,In a prospective, non-interventional, post-marketing study of 2,817 Japanese pediatric and adult cardiac surgery patients, Mallinckrodt’s INOflo® was evaluated for perioperative pulmonary hypertension. The study found that mean inspired nitrogen dioxide concentrations remained \(\le 0.5\) ppm throughout 48 hours of treatment.
Market Challenges Analysis:
High Treatment Costs and Limited Accessibility in Emerging Markets:
The inhaled nitric oxide delivery system market faces challenges due to high treatment costs and limited availability in developing regions. Specialized equipment, continuous supply needs, and maintenance requirements create financial strain for hospitals. Limited reimbursement policies further restrict access, particularly in low-resource settings. It reduces adoption among healthcare facilities that operate under strict budget constraints. The disparity between advanced healthcare systems and underserved markets slows overall global growth. Expanding cost-effective solutions and broader reimbursement frameworks remain critical to address these barriers.
Safety Concerns and Strict Regulatory Compliance Requirements:
Safety issues related to dosage precision and potential side effects present another challenge for widespread adoption. Regulatory bodies impose strict approval standards for both delivery systems and therapeutic use. Lengthy approval timelines and complex compliance demands delay product launches and market expansion. It increases the burden on manufacturers to maintain continuous innovation while meeting stringent quality benchmarks. Training needs for clinicians also limit adoption where expertise is lacking. The inhaled nitric oxide delivery system market must overcome these challenges to sustain long-term adoption and growth.
Market Opportunities:
Expansion into Emerging Healthcare Markets and Growing Infrastructure Investments:
The inhaled nitric oxide delivery system market holds significant opportunities in emerging economies where healthcare infrastructure is rapidly expanding. Rising investments in neonatal and critical care units across Asia-Pacific, Latin America, and the Middle East create strong demand potential. Governments and private players are focusing on improving access to advanced respiratory therapies. It opens pathways for manufacturers to introduce cost-effective and scalable solutions tailored for resource-limited settings. Increasing medical tourism in these regions further strengthens market growth prospects. Partnerships with local distributors and healthcare institutions can accelerate adoption and improve availability.
Broadening Clinical Applications and Ongoing Research Initiatives:
Widening therapeutic applications beyond neonatal care present another major opportunity for the inhaled nitric oxide delivery system market. Research into potential use for chronic respiratory diseases, cardiovascular support, and pulmonary complications expands the scope of treatment. Clinical trials continue to demonstrate the safety and efficacy of nitric oxide in diverse conditions. It supports stronger acceptance among healthcare professionals and accelerates regulatory approvals for new indications. The growing emphasis on personalized and non-invasive therapies creates room for innovative product development. Companies that leverage research-driven strategies are well-positioned to capture long-term market growth.
Market Segmentation Analysis:
By Type:
The inhaled nitric oxide delivery system market is segmented into portable and standalone systems. Portable devices are gaining traction due to their flexibility in transport and emergency care. It supports better patient mobility and enhances treatment in resource-limited settings. Standalone systems remain vital in intensive care units, offering precise monitoring and high-capacity performance. Their dominance is supported by established hospital infrastructure and strong integration with ventilator systems.
- For Instance, Bellerophon Therapeutics’ portable INOpulse® system completed enrollment of 145 participants in its Phase III REBUILD study to test for sustained, outpatient pulsed iNO delivery at 45 µg/kg/hr over long-term oxygen therapy. However, the trial failed to meet its primary endpoint, showing no statistically significant benefit over placebo. As a result, Bellerophon ceased operations and was subsequently liquidated
By Application:
Applications include neonatal care, pediatric care, and adult care. Neonatal care holds the largest share due to the rising incidence of hypoxic respiratory failure among newborns. It secures strong demand in specialized neonatal intensive care units. Pediatric and adult applications are growing as clinical use expands in pulmonary hypertension and acute respiratory distress syndrome. Increasing evidence of safety and efficacy broadens adoption in multi-specialty hospitals.
- For Instance, In a 2005 post-hoc analysis of the STRIDE-1 clinical trial, treatment with the drug sitaxsentan resulted in a placebo-subtracted improvement of 65.1 meters in the 6-minute walk distance among a specific subgroup of pulmonary hypertension patients who met traditional enrollment criteria
By Product:
Products include delivery devices and gas cartridges or cylinders. Delivery devices account for significant demand due to their role in precise dosing and patient safety. It ensures accurate administration across neonatal, pediatric, and adult patients. Gas cartridges and cylinders provide the essential supply chain component for therapy. Growth in this segment is supported by rising hospital usage and expanding distribution networks.
Segmentations:
By Type:
- Portable Systems
- Standalone Systems
By Application:
- Neonatal Care
- Pediatric Care
- Adult Care
By Product:
- Delivery Devices
- Gas Cartridges and Cylinders
By Region:
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
North America:
North America held 42% market share in 2024, maintaining its leadership in the inhaled nitric oxide delivery system market. The region benefits from advanced healthcare infrastructure, high awareness of nitric oxide therapies, and strong reimbursement policies. The United States leads demand due to the presence of major manufacturers and a high patient pool requiring critical care. It gains support from ongoing technological innovation and widespread clinical adoption in neonatal and adult respiratory treatments. Growing investments in intensive care facilities further strengthen regional growth. Canada complements this trend with supportive government initiatives and rising focus on neonatal care standards.
Europe:
Europe accounted for 31% market share in 2024, supported by established healthcare systems and strong regulatory frameworks. Germany, France, and the United Kingdom dominate usage with significant adoption in neonatal intensive care units. It benefits from increased funding for advanced therapies and the growing role of nitric oxide in treating pulmonary conditions. Collaborative research across academic institutions and hospitals enhances awareness and accelerates clinical adoption. Favorable reimbursement structures ensure accessibility across public and private healthcare systems. Expansion of pediatric and neonatal care infrastructure continues to drive steady demand in the region.
Asia-Pacific:
Asia-Pacific captured 19% market share in 2024, emerging as the fastest-growing region for inhaled nitric oxide delivery systems. China and India lead growth due to rising healthcare expenditure, large neonatal populations, and rapid urbanization. It gains momentum from government-backed programs expanding access to critical care services. Increasing establishment of advanced neonatal and pediatric facilities boosts demand for effective respiratory therapies. Japan, South Korea, and Southeast Asian countries contribute to the regional outlook with strong technology adoption. The region presents significant opportunities for manufacturers focused on cost-effective and scalable solutions.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- Mallinckrodt Pharmaceuticals
- VERO Biotech
- Getinge AB
- Linde plc
- SLE Ltd.
- Beyond Air, Inc.
- Bellerophon Therapeutics
- Circassia Pharmaceuticals
- Air Liquide Healthcare
- International Biomedical
Competitive Analysis:
The inhaled nitric oxide delivery system market is characterized by the presence of global and regional players competing through innovation and strategic expansion. Key companies include Mallinckrodt Pharmaceuticals, VERO Biotech, Getinge AB, Linde plc, SLE Ltd., Beyond Air, Inc., and Bellerophon Therapeutics. These firms focus on advancing device technology, improving portability, and ensuring precise dosing capabilities to strengthen adoption across neonatal, pediatric, and adult care. It benefits from continuous product launches, regulatory approvals, and collaborations with hospitals and research institutions. Companies also invest in clinical trials to expand therapeutic applications beyond traditional respiratory care. Strategic partnerships and distribution agreements play a vital role in expanding market reach, particularly in emerging economies. Competitive intensity remains high, driven by the need to balance innovation with cost-effectiveness. The market outlook favors companies that combine strong R&D pipelines with efficient supply chains and broad geographic presence.
Recent Developments:
- In August 2025, Mallinckrodt Pharmaceuticals completed its merger with Endo, Inc., forming a global, scaled, and diversified therapeutics leader, with plans to spin off the generics and sterile injectables businesses into Par Health by Q4 2025.
- In May 2025, VERO Biotech announced a strategic partnership and closed a growth equity round co-led by Fifth Wall and Sunriver Capital Partners, with additional participation from Rebuild Capital, aimed at supporting product innovation and accelerating operations.
- In July 2025, Getinge AB entered a strategic partnership with Zimmer Biomet, enabling Zimmer Biomet to distribute Getinge’s Operating Room capital products to Ambulatory Surgery Center customers in the United States, thus enhancing turnkey solutions for surgical and infection control portfolios.
Report Coverage:
The research report offers an in-depth analysis based on Type, Application, Product and Region. It details leading Market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current Market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven Market expansion in recent years. The report also explores Market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on Market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the Market.
Future Outlook:
- Growing demand for inhaled nitric oxide delivery systems will continue due to rising respiratory disorders.
- Adoption in neonatal intensive care units will remain strong, supporting higher survival rates in infants.
- Wider clinical use in cardiology and pulmonary care will expand the scope of applications.
- Technological innovations will focus on portable, compact, and smart systems with advanced monitoring.
- Regulatory approvals for new therapeutic indications will strengthen acceptance among healthcare providers.
- Healthcare investments in emerging economies will create opportunities for broader accessibility and adoption.
- Collaborations between manufacturers, hospitals, and research institutions will accelerate product development and clinical trials.
- Training programs for clinicians will improve safe usage and expand penetration in developing regions.
- Focus on cost-effective and user-friendly solutions will drive adoption in budget-constrained healthcare systems.
- The inhaled nitric oxide delivery system market will evolve with rising emphasis on non-invasive and personalized care.




