Market Overview
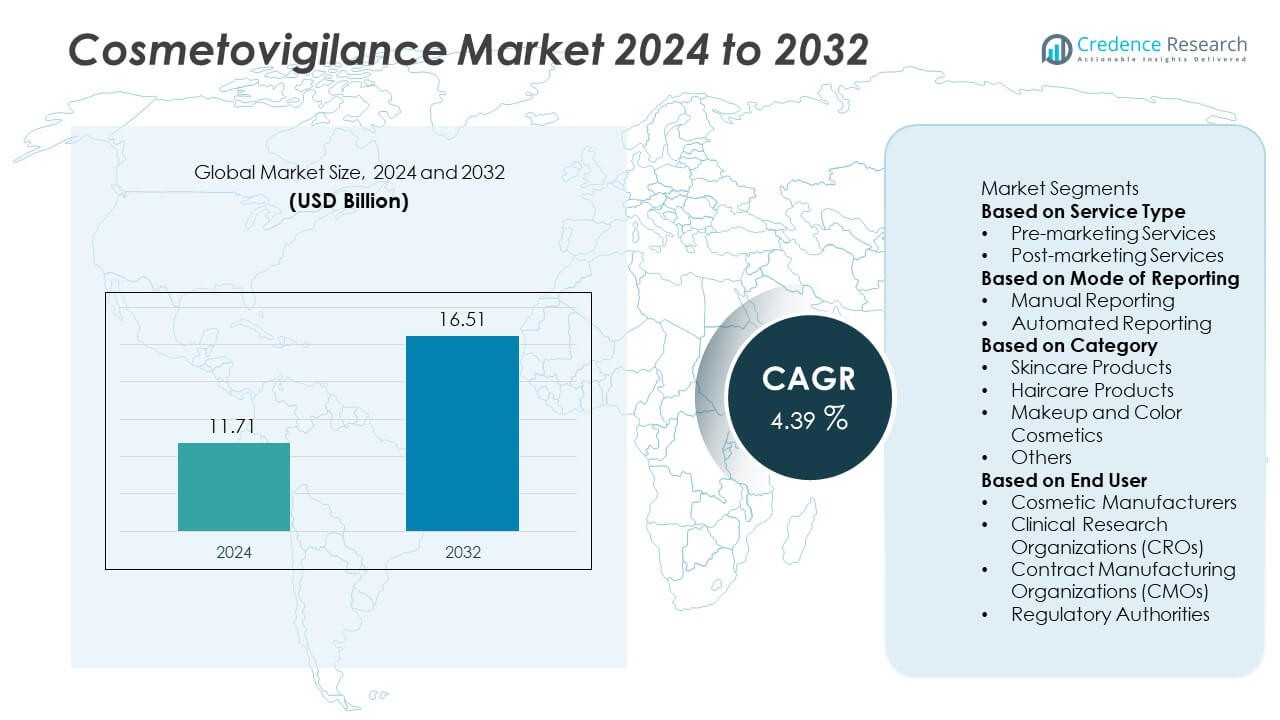
The Cosmetovigilance Market was valued at USD 11.71 billion in 2024 and is projected to reach USD 16.51 billion by 2032, registering a CAGR of 4.39% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Cosmetovigilance Market Size 2024 |
USD 11.71 Billion |
| Cosmetovigilance Market, CAGR |
4.39% |
| Cosmetovigilance Market Size 2032 |
USD 16.51 Billion |
The Cosmetovigilance Market is dominated by key players such as Pharmathen, AxeRegel, Poseidon CRO, Cliantha, PharSafer, iSafety, Skill Pharma, ZEINCRO, FMD K&L, and MSL Solution Providers. These companies lead through robust regulatory expertise, advanced pharmacovigilance systems, and strategic partnerships with cosmetic manufacturers. North America led the market in 2024, holding a 37% share, supported by strong regulatory enforcement from agencies such as the FDA and high adoption of digital safety monitoring solutions. Europe followed with a 33% share, driven by the EU’s strict cosmetic product safety framework, while Asia-Pacific accounted for 26%, fueled by expanding cosmetic production and growing emphasis on consumer safety compliance across developing markets.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The Cosmetovigilance Market was valued at USD 11.71 billion in 2024 and is projected to reach USD 16.51 billion by 2032, expanding at a CAGR of 4.39%.
- Increasing regulatory scrutiny and consumer awareness regarding product safety are driving the adoption of post-marketing surveillance and risk assessment services globally.
- Market trends highlight the integration of AI-based data analytics and automated reporting tools to enhance adverse event tracking and compliance efficiency.
- Key players such as Pharmathen, AxeRegel, and PharSafer are strengthening market presence through advanced reporting systems, regulatory consulting, and partnerships with major cosmetic brands.
- North America led the market with a 37% share in 2024, followed by Europe at 33% and Asia-Pacific at 26%; by service type, post-marketing services dominated with 56% share, supported by expanding compliance requirements and global safety monitoring mandates.
Market Segmentation Analysis:
By Service Type
The post-marketing services segment dominated the Cosmetovigilance Market in 2024, holding a 63% market share. This segment’s leadership is attributed to increasing global emphasis on monitoring adverse events after product commercialization. Regulatory bodies such as the European Medicines Agency (EMA) and the U.S. FDA mandate post-market safety assessments to ensure consumer protection. Cosmetic manufacturers rely on third-party organizations for surveillance, data collection, and regulatory reporting. The growing complexity of international cosmetic regulations and rising public awareness of product safety continue to drive the demand for efficient post-marketing cosmetovigilance systems.
- For instance, PharSafer manages a global database exceeding 1.2 million safety case reports, including adverse event submissions for cosmetic and consumer products. The company employs validated Argus Safety and ArisG platforms for signal detection and compliance reporting across 52 regulatory territories, providing 24/7 pharmacovigilance and cosmetovigilance coverage for multinational brands under EMA and MHRA guidelines.
By Mode of Reporting
The automated reporting segment led the Cosmetovigilance Market in 2024, capturing a 57% share. The dominance stems from the adoption of AI-driven platforms and digital data management systems that enhance accuracy and speed in adverse event reporting. Automation allows real-time monitoring, risk assessment, and integration with regulatory databases. Companies are shifting from traditional manual reporting to automated workflows to improve compliance efficiency and reduce human error. Increasing implementation of cloud-based surveillance tools and digital transformation in cosmetic regulatory operations further support this segment’s growth.
- For instance, the cloud-based EXTEDO SafetyEasy™ solution provides multivigilance capabilities across pharmaceuticals, medical devices, and cosmetics, using AI to streamline case processing. Its CasEasy AI module utilizes Natural Language Processing (NLP) to convert unstructured text from various sources into structured safety case formats, which can significantly reduce manual intake time.
By Category
The skincare products segment accounted for the largest share of 44% in the Cosmetovigilance Market in 2024. Rising consumer use of anti-aging creams, sunscreens, and moisturizers has increased the need for continuous safety monitoring. Skincare products often involve active ingredients that require post-market evaluation for potential reactions or sensitivities. Cosmetic companies are strengthening surveillance systems to detect and manage adverse events efficiently. The segment’s growth is also supported by regulatory focus on ingredient transparency and increasing awareness about dermatological health among consumers worldwide.
Key Growth Drivers
Increasing Regulatory Scrutiny on Cosmetic Safety
Rising global enforcement of cosmetic safety regulations is a major driver of the Cosmetovigilance Market. Regulatory agencies in Europe, North America, and Asia are strengthening post-market surveillance requirements to ensure consumer protection. Companies must now report adverse effects, maintain safety databases, and comply with regional frameworks like EU Regulation 1223/2009. This growing regulatory pressure encourages manufacturers to invest in professional cosmetovigilance systems. The expanding product diversity and cross-border trade of cosmetics further reinforce the need for consistent global safety monitoring mechanisms.
- For instance, AxeRegel is a consulting firm that provides regulatory services, including cosmetovigilance, for pharmaceutical and biotech companies. Their services include evaluating undesirable effects and reporting them to relevant authorities.
Rising Consumer Awareness and Demand for Safe Cosmetics
Consumers are increasingly prioritizing product safety and ingredient transparency, fueling demand for cosmetovigilance services. Social media exposure and health awareness campaigns have made customers more vigilant about product reactions and chemical content. As a result, brands are adopting stricter safety assessments and complaint monitoring systems. Growing adoption of natural and dermatologically tested cosmetics also requires continuous safety evaluation. This consumer-driven shift toward accountability and trust is propelling the market for robust post-marketing surveillance and adverse event reporting systems.
- For instance, Cliantha Research conducts dermatology and consumer research, including assessments of product safety and efficacy, to support product claims for skincare, personal care, and other formulations. The company has facilities in India and North America and offers services that include consumer in-use tests, which track consumer perception and potential adverse skin reactions to cosmetic products.
Expanding Global Cosmetics Industry
The rapid expansion of the cosmetics and personal care sector is creating sustained demand for cosmetovigilance solutions. New product launches across skincare, makeup, and haircare categories increase the risk of potential side effects, necessitating structured safety monitoring. Multinational brands rely on regional reporting networks and AI-based systems to track adverse reactions efficiently. Emerging markets in Asia-Pacific and Latin America are witnessing heightened cosmetic consumption, further increasing safety reporting volumes. As global production scales up, effective cosmetovigilance becomes integral to maintaining compliance and brand integrity.
Key Trends & Opportunities
Integration of AI and Automation in Safety Monitoring
Artificial intelligence and automation are transforming cosmetovigilance by improving data accuracy and speed. Automated systems enable real-time reporting, predictive analysis, and streamlined case management. AI tools analyze large datasets to identify adverse reaction trends early. Companies are integrating machine learning with pharmacovigilance frameworks to enhance decision-making. This technological shift is reducing operational costs and improving compliance. The rising adoption of digital health ecosystems offers significant opportunities for automated cosmetovigilance platforms and cloud-based monitoring systems.
- For instance, iSafety Systems, acquired by ProPharma Group, offers pharmacovigilance services and safety solutions to pharmaceutical, biotechnology, and medical device companies. These services include case reporting, signal detection, and risk management.
Rising Adoption of Outsourced Cosmetovigilance Services
Many cosmetic manufacturers are outsourcing cosmetovigilance activities to specialized service providers for better regulatory compliance. Third-party firms offer expertise in safety data management, risk assessment, and adverse event reporting. This approach reduces internal costs and ensures adherence to complex regional regulations. The increasing globalization of cosmetic trade and the expansion of product portfolios make outsourcing an attractive strategy. Partnerships between manufacturers and contract research organizations are expected to grow, creating opportunities for niche service providers in the cosmetovigilance domain.
- For instance, ZEINCRO, now part of the Excelya Group, is a Contract Research Organization (CRO) that provides clinical trial services and pharmacovigilance to pharmaceutical, biotechnology, and medical device companies across more than 20 countries, with a focus on Central and South-Eastern Europe.
Key Challenges
Complex and Fragmented Global Regulatory Landscape
Differing safety and reporting regulations across countries pose significant challenges for cosmetic companies. Each region maintains distinct standards for adverse event classification, documentation, and timelines. This inconsistency complicates global compliance and delays data harmonization. Smaller brands often lack the resources to manage multiple regulatory frameworks efficiently. Harmonizing safety reporting procedures across global markets remains a key hurdle for the industry and demands closer collaboration between authorities and manufacturers.
High Implementation Costs and Limited Awareness in Developing Markets
Setting up advanced cosmetovigilance systems involves high costs related to technology, training, and regulatory consultation. Developing countries face barriers such as limited awareness, weak enforcement, and inadequate infrastructure for safety reporting. Many small and mid-sized enterprises struggle to justify investment in full-scale monitoring systems. Additionally, the lack of skilled personnel and standardized reporting mechanisms further constrains adoption. Addressing these cost and knowledge gaps is essential to expanding cosmetovigilance practices globally.
Regional Analysis
North America
North America held a 35% market share in the Cosmetovigilance Market in 2024. The region’s leadership is driven by strong regulatory enforcement, advanced healthcare infrastructure, and high consumer awareness of product safety. The United States dominates due to stringent FDA oversight on post-market cosmetic surveillance and growing demand for clean-label products. Increasing adoption of automated reporting tools and integration of AI in safety assessment further strengthen market presence. The rise of cosmetic product innovations and frequent new launches continue to fuel demand for robust safety monitoring systems across the region.
Europe
Europe accounted for a 32% share of the Cosmetovigilance Market in 2024, supported by the region’s strict cosmetic safety regulations under EU Regulation 1223/2009. Countries such as Germany, France, and the United Kingdom are leading adopters of comprehensive post-marketing surveillance systems. The region’s emphasis on consumer health protection and transparency drives demand for advanced reporting and safety evaluation frameworks. Strong collaborations between regulatory bodies and industry players promote effective compliance. The growing trend of sustainable and natural cosmetics also encourages proactive monitoring of ingredient safety across the European market.
Asia-Pacific
Asia-Pacific captured a 27% market share in the Cosmetovigilance Market in 2024. Rapid expansion of the cosmetics industry in China, Japan, South Korea, and India is fueling the need for efficient safety monitoring systems. Increasing regulatory reforms, particularly in China’s cosmetics supervision framework, are encouraging manufacturers to adopt structured reporting mechanisms. Rising consumer awareness and digital transformation in healthcare are promoting wider adoption of automated cosmetovigilance platforms. The region’s growing export-oriented cosmetic production base and government-led product safety initiatives continue to enhance market growth and compliance capabilities.
Latin America
Latin America held a 4% share of the Cosmetovigilance Market in 2024. Growth is supported by increasing cosmetic consumption and expanding regulatory initiatives in Brazil, Mexico, and Argentina. National health agencies are strengthening post-marketing surveillance requirements to align with international standards. Rising consumer concerns about adverse effects and counterfeit products drive market adoption. Local manufacturers are partnering with global cosmetovigilance providers to improve reporting accuracy and compliance. The growing beauty industry, coupled with digitalization in safety monitoring, supports gradual market expansion across Latin America.
Middle East & Africa
The Middle East & Africa region accounted for a 2% market share in the Cosmetovigilance Market in 2024. Market growth is driven by improving regulatory frameworks and growing awareness of cosmetic safety standards. The Gulf Cooperation Council (GCC) countries, led by Saudi Arabia and the United Arab Emirates, are enforcing stricter post-market monitoring of cosmetic products. In Africa, increasing urbanization and the expansion of local cosmetic manufacturing are enhancing awareness of safety compliance. Government efforts to align cosmetic regulations with global norms are further supporting market development in this emerging region.
Market Segmentations:
By Service Type
- Pre-marketing Services
- Post-marketing Services
By Mode of Reporting
- Manual Reporting
- Automated Reporting
By Category
- Skincare Products
- Haircare Products
- Makeup and Color Cosmetics
- Others
By End User
- Cosmetic Manufacturers
- Clinical Research Organizations (CROs)
- Contract Manufacturing Organizations (CMOs)
- Regulatory Authorities
By Geography
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Competitive Landscape
Competitive landscape of the Cosmetovigilance Market features key players such as Pharmathen, AxeRegel, Poseidon CRO, Cliantha, PharSafer, iSafety, Skill Pharma, ZEINCRO, FMD K&L, and MSL Solution Providers. These companies maintain strong market positions through specialized regulatory expertise, global pharmacovigilance networks, and advanced data management systems. Leading service providers focus on developing AI-driven reporting platforms and automated safety monitoring tools to enhance compliance with evolving EU and FDA cosmetic regulations. Strategic collaborations with cosmetic manufacturers and contract research organizations (CROs) are expanding service reach. The growing demand for post-marketing surveillance, coupled with increasing emphasis on consumer safety and adverse event documentation, is driving competition. Companies are investing in real-time analytics, automated data capture, and regulatory intelligence platforms to ensure efficient and compliant safety reporting. This competitive environment encourages continuous innovation and expansion into emerging markets, particularly in Asia-Pacific and Latin America, where cosmetic regulations are becoming increasingly stringent.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
Recent Developments
- In June 2025, AxeRegel expanded its cosmetic-regulation advisory services, emphasizing cosmetovigilance and authoring guidance on reporting undesirable effects under EU Cosmetics Regulation (EC) No 1223/2009.
- In 2025, PharSafer enhanced its global safety operations by integrating new electronic reporting systems that allow real-time tracking of adverse cosmetic events from multiple regions, ensuring faster compliance with EMA and FDA post-marketing safety requirements.
- In 2024, Cliantha Research announced the expansion of its Consumer Clinical Research division in Mississauga, Canada, to include safety and efficacy studies for cosmetics and personal care formulations, supporting over 7,200 completed trials across its global sites.
Report Coverage
The research report offers an in-depth analysis based on Service Type, Mode of Reporting, Category, End User and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market will grow steadily with increasing global focus on cosmetic product safety compliance.
- Post-marketing surveillance will continue to dominate as regulatory authorities strengthen safety reporting standards.
- Automation and AI will streamline data collection, signal detection, and adverse event analysis.
- Collaboration between cosmetic manufacturers and regulatory outsourcing firms will expand globally.
- Asia-Pacific will emerge as a high-growth region driven by expanding cosmetics manufacturing and export activities.
- Digital reporting tools and centralized safety databases will enhance transparency and response speed.
- Demand for skilled pharmacovigilance and cosmetovigilance professionals will rise across contract research organizations.
- Regulatory harmonization across regions will promote unified safety assessment frameworks.
- Increasing use of real-world data and predictive analytics will improve risk evaluation accuracy.
- Investment in advanced compliance software and cloud-based reporting systems will reshape future market operations.




