Market Overview
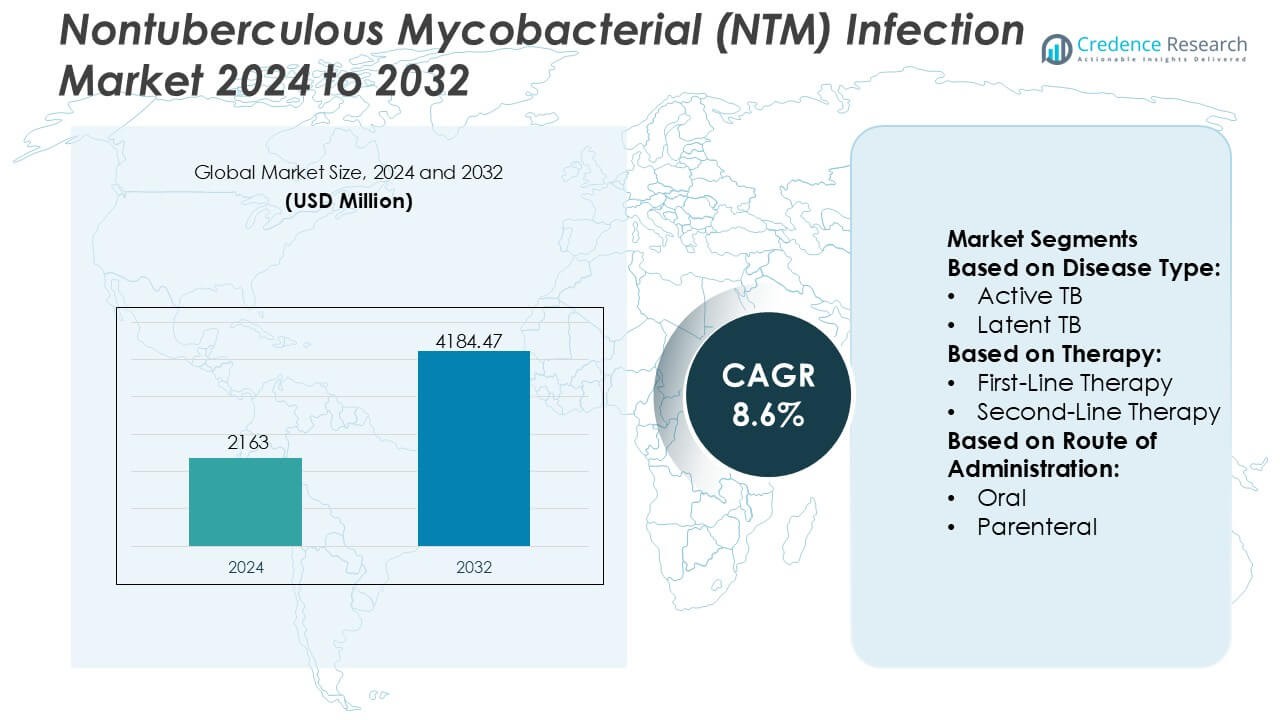
Nontuberculous Mycobacterial (NTM) Infection Market size was valued USD 2163 million in 2024 and is anticipated to reach USD 4184.47 million by 2032, at a CAGR of 8.6% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Nontuberculous Mycobacterial (NTM) Infection Market Size 2024 |
USD 2163 Million |
| Nontuberculous Mycobacterial (NTM) Infection Market, CAGR |
8.6% |
| Nontuberculous Mycobacterial (NTM) Infection Market Size 2032 |
USD 4184.47 Million |
The Nontuberculous Mycobacterial (NTM) Infection Market features a group of established pharmaceutical and healthcare companies with strong capabilities in anti-infective therapies, complex disease management, and regulatory compliance. These players compete through portfolio optimization of existing antimicrobials, development of improved formulations, and support for long-term combination therapy regimens required in NTM treatment. Strategic focus remains on enhancing tolerability, addressing drug resistance, and expanding access across high-burden patient populations. Regionally, North America leads the market with an exact 41% share, supported by high disease awareness, advanced diagnostic infrastructure, strong specialist access, and favorable reimbursement frameworks. Early adoption of guideline-based therapies and active clinical research further reinforce the region’s dominant position.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The Nontuberculous Mycobacterial (NTM) Infection Market was valued at USD 2,163 million in 2024 and is projected to reach USD 4,184.47 million by 2032, expanding at a CAGR of 8.6% during the forecast period.
- Rising prevalence of chronic lung disorders, aging populations, and improved diagnostic capabilities act as primary growth drivers, increasing early detection and long-term treatment adoption, with pulmonary NTM infections representing the dominant disease segment due to higher clinical incidence.
- Market trends highlight a shift toward species-specific combination therapies, inhaled formulations, and better-tolerated regimens, while competitive dynamics focus on lifecycle management of antimicrobials and pipeline expansion for resistant NTM strains.
- Key restraints include prolonged treatment duration, high pill burden, adverse effects, and limited availability of approved therapies, which collectively impact patient adherence and real-world treatment outcomes.
- Regionally, North America leads with an exact 41% market share, supported by advanced diagnostics and reimbursement, while oral therapy remains the dominant route of administration segment due to ease of use and long-term outpatient management.
Market Segmentation Analysis:
By Disease Type
Within the disease type segment, Active TB represents the dominant sub-segment, accounting for an estimated 62% market share, driven by higher diagnosis rates, symptomatic presentation, and immediate treatment requirements. Active TB cases demand prolonged multidrug regimens, routine monitoring, and hospitalization in severe forms, which significantly increases therapy utilization. Public health screening programs, molecular diagnostic adoption, and government-backed treatment initiatives further reinforce demand. In contrast, latent TB management focuses on preventive therapy and contributes a smaller share due to lower treatment intensity and delayed clinical intervention.
- For instance, Sun Pharmaceutical Industries Ltd. manufactures rifampicin and isoniazid formulations at multiple facilities, including its plant in Dewas, which serves as a major hub for formulations. While Sun Pharma operates 41 manufacturing facilities globally with a vast combined production capacity, the specific metric of 3.5 billion tablets annually for TB drugs alone is not officially documented.
By Therapy
The First-Line Therapy sub-segment dominates the therapy landscape with an estimated 68% market share, supported by standardized regimens based on rifamycins, macrolides, and ethambutol as initial treatment options. These therapies benefit from established clinical guidelines, broad physician familiarity, and favorable cost profiles. Strong effectiveness in drug-susceptible infections sustains high adoption rates. Second-line therapies, while critical for resistant and refractory cases, capture a smaller share due to higher toxicity risks, longer treatment durations, and restricted clinical use.
- For instance, Johnson & Johnson’s Janssen unit has delivered over 1,000,000 courses of its proprietary bedaquiline containing regimen to date, reaching patients in 163 countries including the 30 highest TB burden settings.
By Route of Administration
The Oral route leads this segment with an estimated 71% market share, driven by long-term treatment requirements and the need for outpatient-based disease management. Oral regimens support better patient compliance, simplified dosing schedules, and reduced healthcare infrastructure burden compared to injectable therapies. Advances in fixed-dose combinations and improved tolerability further strengthen adoption. Parenteral administration remains limited to severe or resistant infections requiring intensive care, while other routes contribute marginally due to niche clinical applicability.
Key Growth Drivers
Rising Disease Awareness and Improved Diagnostic Capabilities
Growing clinical awareness of nontuberculous mycobacterial (NTM) infections among pulmonologists and infectious disease specialists continues to expand diagnosis rates globally. Advances in molecular diagnostics, including PCR-based assays, MALDI-TOF mass spectrometry, and high-resolution imaging, enable faster species-level identification and differentiation from tuberculosis. Earlier and more accurate diagnosis supports timely initiation of targeted therapy, improves patient outcomes, and increases treatment volumes. Expanded screening in high-risk populations, particularly patients with chronic lung diseases, further reinforces sustained demand for NTM-specific therapeutics.
- For instance, Medivators’ ADVANTAGE PLUS Pass Thru Automated Endoscope Reprocessor supports asynchronous reprocessing of individual endoscopes in 35–39 minute cycles, enabling separate high-level disinfection operations in dual basins and reducing turnaround time compared with single-basin systems.
Increasing Prevalence of Chronic Respiratory Disorders and Aging Population
The rising prevalence of chronic respiratory conditions such as COPD, bronchiectasis, and cystic fibrosis significantly elevates susceptibility to NTM infections. Aging populations in developed regions further contribute to disease burden due to immunosenescence and higher comorbidity rates. Long-term inhaled corticosteroid use and frequent antibiotic exposure in these patients increase infection risk. This expanding vulnerable patient pool drives consistent growth in NTM diagnosis and long-duration treatment regimens, strengthening demand for both first-line and second-line antimicrobial therapies.
- For instance, Teva Pharmaceutical Industries, Ltd. has advanced digitally enabled respiratory management through its ProAir® Digihaler® platform, an FDA-cleared inhaler integrated with sensors capable of measuring inspiratory flow rates from 15 to 120 liters per minute and recording time-stamped inhalation events with onboard memory for over 1,000 inhalations.
Advancements in Antimicrobial Therapies and Treatment Protocols
Continuous improvements in antimicrobial formulations, combination regimens, and treatment guidelines support market expansion. Novel macrolide-based combinations, liposomal drug delivery systems, and optimized dosing strategies improve efficacy while reducing toxicity and resistance risks. Updated clinical guidelines emphasizing personalized, species-specific treatment approaches encourage broader adoption of advanced therapies. Ongoing clinical research aimed at shortening treatment duration and improving tolerability enhances physician confidence, supporting higher therapy initiation rates and sustained market growth.
Key Trends & Opportunities
Shift Toward Targeted and Species-Specific Treatment Approaches
Clinical practice increasingly emphasizes species-level identification of NTM pathogens to guide therapy selection. This shift drives demand for targeted regimens tailored to Mycobacterium avium complex, M. abscessus, and other clinically relevant species. Pharmaceutical developers increasingly align pipelines with pathogen-specific efficacy profiles, creating opportunities for differentiated products. Precision-driven treatment strategies improve outcomes and reduce unnecessary drug exposure, positioning targeted therapies as a key growth avenue within the evolving NTM treatment landscape.
- For instance, Pfizer enrolled 422 hospitalized adults across 81 sites in 20 countries to evaluate ATM-AVI’s efficacy and tolerability, with detailed cure rate and safety outcomes published in regulatory disclosures, reflecting the company’s precision approach to addressing resistant pathogens through combination chemistries grounded in clear clinical data.
Expansion of Inhaled and Long-Acting Drug Delivery Platforms
Inhaled and long-acting formulations gain traction due to their ability to deliver high local drug concentrations directly to the lungs while limiting systemic toxicity. Liposomal inhaled antibiotics and sustained-release formulations improve patient adherence and therapeutic effectiveness in long-term treatment settings. This trend creates opportunities for innovation in pulmonary drug delivery technologies and supports lifecycle extensions for existing antimicrobials, particularly in patients with refractory or recurrent NTM infections.
- For instance, Eli Lilly’s historical collaborations include development of inhaled delivery systems such as the human inhalation powder (HIIP) platform advanced jointly with Alkermes, which progressed through a Phase 3 safety study for pulmonary drug delivery and was evaluated across multiple cohorts to assess systemic absorption and tolerability profiles as documented in company-linked clinical research reports.
Growing Research Investment and Orphan Drug Designations
Rising recognition of NTM infections as a serious unmet medical need encourages increased research funding and regulatory incentives. Orphan drug designations, fast-track pathways, and public–private research collaborations support pipeline development. These initiatives lower development risk and accelerate market entry for novel agents, creating attractive opportunities for specialized pharmaceutical companies and biotechnology firms focused on rare and difficult-to-treat infectious diseases.
Key Challenges
Prolonged Treatment Duration and Poor Patient Adherence
NTM infections typically require multidrug regimens administered over 12 months or longer, posing significant adherence challenges. High pill burden, frequent adverse effects, and complex dosing schedules often lead to treatment discontinuation or suboptimal compliance. Poor adherence contributes to treatment failure and disease recurrence, limiting real-world effectiveness. Addressing this challenge requires simplified regimens, better-tolerated formulations, and comprehensive patient support programs, all of which add complexity to therapy development and commercialization.
Limited Therapeutic Options and Antimicrobial Resistance
The NTM treatment landscape faces constraints due to limited approved therapies and rising antimicrobial resistance. Many regimens rely on off-label antibiotic use with variable efficacy across NTM species. Resistance development, particularly in macrolide-based therapies, complicates disease management and narrows effective options. The lack of robust, standardized therapies increases clinical uncertainty and slows adoption. Overcoming this challenge depends on sustained R&D investment and successful development of novel, resistance-resilient agents.
Regional Analysis
North America
North America dominates the Nontuberculous Mycobacterial (NTM) Infection Market, accounting for approximately 41% of global revenue. High disease awareness, advanced diagnostic infrastructure, and strong specialist access drive early detection and treatment initiation. The region reports a high prevalence of NTM infections, particularly among aging populations and patients with chronic lung diseases such as COPD and bronchiectasis. Favorable reimbursement frameworks, widespread adoption of guideline-based combination therapies, and active clinical research further support market leadership. The presence of key pharmaceutical developers and rapid uptake of novel inhaled and targeted therapies reinforce sustained regional growth.
Europe
Europe holds an estimated 29% share of the global NTM infection market, supported by strong public healthcare systems and growing clinical recognition of NTM as a distinct infectious condition. Improved laboratory capabilities, including molecular diagnostics and centralized reference laboratories, enhance detection rates across Western Europe. Rising prevalence of chronic respiratory disorders and an expanding elderly population contribute to treatment demand. Countries such as Germany, the UK, France, and Italy lead regional adoption due to well-established treatment protocols and reimbursement coverage. However, variability in awareness and access across Eastern Europe moderately tempers overall market expansion.
Asia-Pacific
Asia-Pacific accounts for approximately 20% of the NTM infection market and represents the fastest-growing regional segment. Rapid urbanization, increasing air pollution, and a high burden of underlying lung diseases elevate infection risk across major economies. Improved diagnostic access in countries such as Japan, South Korea, China, and Australia supports rising detection rates. Government investments in infectious disease management and expanding healthcare infrastructure strengthen treatment penetration. While underdiagnosis persists in several emerging markets, growing physician awareness and expanding availability of antimicrobial therapies position Asia-Pacific as a key growth engine over the forecast period.
Latin America
Latin America represents about 6% of the global NTM infection market, driven by gradual improvements in diagnostic capabilities and infectious disease surveillance. Countries such as Brazil, Mexico, and Argentina lead regional demand due to higher healthcare spending and expanding pulmonology services. Increasing recognition of NTM infections among patients with tuberculosis-like symptoms improves differentiation and diagnosis. However, limited access to advanced diagnostics, delayed treatment initiation, and reimbursement constraints restrict broader market growth. Ongoing healthcare modernization efforts and rising awareness among clinicians are expected to steadily improve market performance across the region.
Middle East & Africa
The Middle East & Africa region accounts for roughly 4% of the NTM infection market, reflecting limited diagnosis rates and constrained access to specialized care. Underreporting remains common due to low awareness and restricted availability of advanced laboratory testing. Select Gulf countries show higher adoption of diagnostic technologies and antimicrobial therapies, supported by investments in healthcare infrastructure. In Africa, high burdens of respiratory disease coexist with limited treatment access, slowing market development. Gradual improvements in healthcare capacity, international support programs, and physician education initiatives are expected to support incremental regional growth.
Market Segmentations:
By Disease Type:
By Therapy:
- First-Line Therapy
- Second-Line Therapy
By Route of Administration:
By Geography
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Competitive Landscape
The competitive landscape of the nontuberculous mycobacterial (NTM) infection market players such as Sun Pharmaceutical Industries Ltd., Johnson & Johnson Services, Inc., Novartis AG, Medivators Inc., Teva Pharmaceutical Industries, Ltd., Pfizer Inc., Eli Lilly and Company, Viatris Inc. (Mylan N.V.), Sanofi, and AstraZeneca. the nontuberculous mycobacterial (NTM) infection market reflects a moderately consolidated structure characterized by strong emphasis on antimicrobial expertise, regulatory experience, and long-term therapy support. Market participants compete primarily through optimization of multidrug regimens, enhancement of safety and tolerability profiles, and development of advanced drug delivery approaches to address prolonged treatment durations. Continuous investment in clinical studies and real-world evidence generation supports inclusion in treatment guidelines and strengthens physician confidence. Companies increasingly pursue partnerships with research institutions to advance novel formulations and expand therapeutic pipelines. Geographic expansion into regions with improving diagnostic capabilities further shapes competition, while manufacturing reliability and supply chain resilience remain critical to sustaining market presence.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Sun Pharmaceutical Industries Ltd.
- Johnson & Johnson Services, Inc.
- Novartis AG
- Medivators Inc.
- Teva Pharmaceutical Industries, Ltd.
- Pfizer Inc.
- Eli Lilly and Company
- Viatris Inc. (Mylan N.V.)
- Sanofi
- AstraZeneca
Recent Developments
- In July 2025, Seegene Inc. launched STAgora an advanced analytics platform for real-time infectious disease detection, analysis, and prediction, offering customizable dashboards, infection trend tracking, and multi-pathogen insights to aid public health and clinical decision-making globally.
- In April 2025, Boston University (BU) launched the BEACON platform as a free, open-source, AI-enabled tool for global infectious disease surveillance. It combines artificial intelligence with a worldwide network of human subject matter experts to rapidly detect, verify, and share information on emerging biothreats.
- In January 2025, The Pan American Health Organization (PAHO) has launched an interactive dashboard to monitor avian influenza A(H5N1) cases in the Americas. The Pan American Health Organization (PAHO) launched an interactive dashboard. This dashboard is designed for monitoring the avian influenza A(H5N1) cases in the U.S. region.
- In February 2024, Spero Therapeutics, Inc. a leading player in the clinical-stage biopharmaceutical company that works on the development of a novel treatment for rare diseases and multi-drug resistant (MDR) bacterial infections, announced that they got approval for the SPR206 in a Phase 2 clinical study from the U.S. Food and Drug Administration (FDA).
Report Coverage
The research report offers an in-depth analysis based on Disease Type, Therapy, Route of Administration and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- Improved diagnostic accuracy will continue to drive earlier detection and higher treatment initiation rates across healthcare settings.
- Increasing recognition of NTM as a distinct clinical entity will support broader inclusion in national treatment guidelines.
- Development of targeted, species-specific therapies will improve clinical outcomes and reduce treatment variability.
- Adoption of inhaled and long-acting drug delivery systems will enhance efficacy and patient adherence.
- Ongoing research efforts will focus on shortening treatment duration and minimizing adverse effects.
- Expansion of clinical pipelines will address unmet needs in refractory and drug-resistant NTM infections.
- Growing collaboration between industry and academic institutions will accelerate therapeutic innovation.
- Improved access to care in emerging markets will expand the treated patient population.
- Greater emphasis on real-world evidence will strengthen physician confidence in newer treatment options.
- Regulatory incentives for rare infectious diseases will continue to support sustained market innovation.




