| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
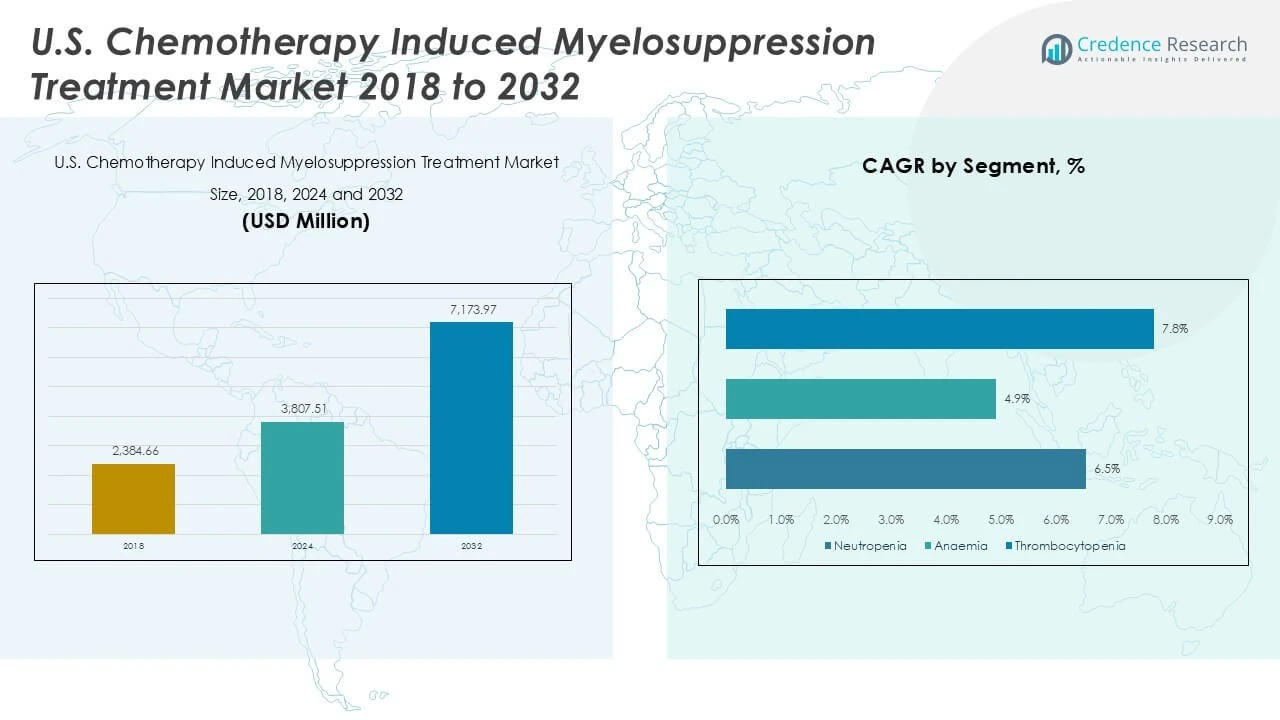
| U.S. Chemotherapy Induced Myelosuppression Treatment Market Size 2024 |
USD 3,807.51 Million |
| U.S. Chemotherapy Induced Myelosuppression Treatment Market, CAGR |
7.67% |
| U.S. Chemotherapy Induced Myelosuppression Treatment Market Size 2032 |
USD 7,173.97 Million |
Market Overview
The U.S. Chemotherapy Induced Myelosuppression Treatment Market is projected to grow from USD 3,807.51 million in 2024 to an estimated USD 7,173.97 million by 2032, with a compound annual growth rate (CAGR) of 7.67% from 2025 to 2032.
Key drivers of market expansion include the growing incidence of cancer, advancements in supportive care therapies, and the development of targeted treatments. Innovations such as granulocyte colony-stimulating factors (G-CSFs), erythropoiesis-stimulating agents (ESAs), and thrombopoietic agents are enhancing the management of chemotherapy-induced myelosuppression. Additionally, the shift towards personalized medicine and the increasing focus on patient-centered care are contributing to the demand for more effective and tailored treatment options.
Geographically, North America leads the chemotherapy-induced myelosuppression treatment market, owing to its advanced healthcare infrastructure, high cancer prevalence, and significant adoption of innovative therapies. The United States, in particular, accounts for a substantial share of the market. Key players in this space include Amgen Inc., Pfizer Inc., Novartis AG, Teva Pharmaceutical Industries Ltd., and Janssen Global Services LLC. These companies are at the forefront of developing and commercializing therapies that address the challenges associated with chemotherapy-induced myelosuppression.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The U.S. Chemotherapy Induced Myelosuppression Treatment Market is projected to grow from USD 3,807.51 million in 2024 to USD 7,173.97 million by 2032, with a CAGR of 7.67%.
- Key drivers include the rising cancer incidence, increased adoption of chemotherapy, and advancements in supportive care therapies, enhancing patient outcomes.
- High treatment costs and varying insurance coverage may limit patient access to advanced therapies, hindering market expansion.
- The growing emphasis on targeted therapies for neutropenia, anemia, and thrombocytopenia is improving treatment efficacy and contributing to market growth.
- North America, especially the U.S., leads the market, with a high concentration of cancer treatment centers and advanced healthcare infrastructure.
- Innovations in personalized medicine and AI-driven treatment models are transforming how chemotherapy-induced myelosuppression is managed.
- Major players like Amgen Inc., Pfizer Inc., and Novartis AG dominate the market by developing and commercializing therapies addressing myelosuppression challenges.
Market Drivers
Rising Cancer Incidence Fueling Demand for Effective Myelosuppression Treatments
The increasing prevalence of cancer in the United States significantly drives the U.S. Chemotherapy Induced Myelosuppression Treatment Market. Growing patient populations undergoing chemotherapy treatments directly contribute to a higher occurrence of myelosuppression. This condition often leads to complications such as neutropenia, anemia, and thrombocytopenia, which can delay or interrupt cancer therapies. The need for effective supportive care treatments to manage these side effects becomes critical in maintaining treatment schedules and improving patient outcomes. The escalating cancer burden therefore amplifies the demand for innovative and reliable therapies that mitigate chemotherapy-induced hematological toxicities.
- For instance, chemotherapy-induced myelosuppression affects a significant portion of patients, requiring specialized supportive care interventions.
Advancements in Supportive Care Therapies Enhancing Market Growth
Therapeutic innovations in supportive care contribute substantially to market expansion. Development of granulocyte colony-stimulating factors (G-CSFs), erythropoiesis-stimulating agents (ESAs), and thrombopoietic agents has improved the management of chemotherapy-induced complications. These therapies aid in stimulating bone marrow recovery and reduce the risk of infections and bleeding episodes. It promotes better tolerance to chemotherapy regimens and reduces hospitalization rates. The continuous pipeline of novel agents and biosimilars further strengthens the market’s growth prospects by broadening treatment options available to healthcare providers and patients.
- For instance, biosimilar adoption in oncology has expanded treatment accessibility, lowering costs while maintaining efficacy.
Increasing Adoption of Personalized Medicine in Cancer Treatment
The U.S. Chemotherapy Induced Myelosuppression Treatment Market benefits from the growing trend toward personalized medicine in oncology. Tailoring treatment protocols to individual patient profiles allows for optimized management of side effects, including myelosuppression. Personalized approaches enable precise dosing and selection of supportive therapies, reducing toxicity risks. It also improves the overall effectiveness of cancer treatments by minimizing treatment interruptions. The integration of genetic and biomarker testing in clinical practice supports this shift, increasing the demand for customizable myelosuppression management solutions.
Healthcare Infrastructure and Awareness Driving Market Expansion
Robust healthcare infrastructure and growing awareness among healthcare professionals and patients contribute to the market’s positive trajectory. Well-established oncology centers and widespread access to advanced diagnostic tools facilitate early detection and management of myelosuppression. Education on the importance of supportive care promotes timely intervention, reducing complications related to chemotherapy. Increased reimbursement policies for supportive therapies encourage their utilization, fostering market growth. Together, these factors enhance the overall quality of cancer care and stimulate demand within the U.S. Chemotherapy Induced Myelosuppression Treatment Market.
Market Trends
Development of Targeted Therapies and Novel Agents
The U.S. Chemotherapy Induced Myelosuppression Treatment Market is experiencing a significant shift towards the development of targeted therapies and novel agents aimed at mitigating chemotherapy-induced myelosuppression. One notable example is trilaciclib (Cosela), a first-in-class cyclin-dependent kinase (CDK) 4/6 inhibitor approved by the FDA in 2021. Trilaciclib has demonstrated efficacy in reducing chemotherapy-induced bone marrow suppression, thereby decreasing the incidence of neutropenia and the need for supportive care in patients undergoing chemotherapy for small cell lung cancer. This advancement underscores the growing emphasis on developing therapies that specifically target the mechanisms underlying myelosuppression, offering patients improved outcomes and quality of life.
- For instance, a study analyzing real-world data from community cancer care providers found that 60.9% of chemotherapy-treated patients experienced grade 3 myelosuppressive adverse events, highlighting the need for targeted therapies.
Integration of Artificial Intelligence in Predictive Modeling
The integration of artificial intelligence (AI) into predictive modeling is emerging as a transformative trend in the management of chemotherapy-induced myelosuppression. Recent studies have explored the use of hybrid mechanistic and data-driven models to predict platelet dynamics during chemotherapy. These models combine traditional pharmacokinetic approaches with machine learning algorithms, such as gated recurrent units, to enhance the accuracy of predictions regarding hematologic recovery. By leveraging patient-specific data, AI-driven models can provide personalized forecasts, enabling clinicians to tailor treatment plans more effectively and anticipate potential complications associated with myelosuppression.
- For instance, a machine learning-based prediction model developed for chemotherapy-induced myelosuppression in children with Wilms’ tumor analyzed 1,433 chemotherapy cycles to enhance early preventive management.
Advancements in Personalized Medicine Approaches
Personalized medicine is gaining traction in the management of chemotherapy-induced myelosuppression, with an increasing focus on tailoring treatments to individual patient profiles. This approach involves utilizing genetic testing and biomarker identification to customize treatment strategies, optimizing efficacy, and minimizing adverse effects. By understanding the unique genetic makeup and tumor characteristics of patients, healthcare providers can select the most appropriate therapies, including supportive care interventions, to manage myelosuppression more effectively. The shift towards personalized medicine not only enhances treatment outcomes but also contributes to the overall advancement of oncology care.
Expansion of Biosimilars and Cost-Effective Treatment Options
The introduction and adoption of biosimilars are influencing the landscape of the U.S. Chemotherapy Induced Myelosuppression Treatment Market by providing cost-effective alternatives to originator biologics. Biosimilars, which are highly similar to approved reference biologics, offer comparable efficacy and safety profiles, making them accessible options for patients requiring long-term supportive care therapies. The availability of biosimilars can alleviate financial burdens on healthcare systems and patients, particularly in the context of chronic conditions like chemotherapy-induced myelosuppression. This trend is expected to continue as more biosimilars receive regulatory approval, broadening the spectrum of affordable treatment choices in oncology.
Market Challenges
High Treatment Costs and Limited Reimbursement Affecting Market Growth
The U.S. Chemotherapy Induced Myelosuppression Treatment Market faces significant challenges due to the high costs associated with advanced therapies. Innovative treatments, including biologics and novel agents, often come with substantial price tags that limit patient accessibility. Insurance reimbursement policies vary widely and sometimes do not fully cover these therapies, creating financial barriers for many patients. This situation hampers widespread adoption and restricts market expansion. Healthcare providers must balance treatment efficacy with cost-effectiveness, which complicates therapy selection. These economic constraints pose a persistent obstacle to optimizing supportive care for chemotherapy patients.
- For instance, the National Cancer Institute estimated that 1.9 million new cancer cases were diagnosed in the U.S. in 2023, highlighting the growing need for effective supportive care solutions to manage chemotherapy-induced complications
Complexity of Managing Diverse Patient Populations and Side Effects
Managing chemotherapy-induced myelosuppression presents challenges due to the heterogeneous nature of patient populations and the variability of side effects. Individual differences in genetic makeup, cancer types, and treatment regimens complicate the prediction and management of myelosuppression severity. It requires personalized monitoring and adjustments to supportive therapies, increasing clinical complexity and resource demands. Adverse effects from treatments themselves, including immunogenic reactions and drug resistance, further limit therapeutic options. The need for precise and adaptable treatment protocols remains critical to improving patient outcomes while minimizing complications within the U.S. Chemotherapy Induced Myelosuppression Treatment Market.
Market Opportunities
Growing Pipeline of Innovative Therapies and Emerging Treatment Modalities
The U.S. Chemotherapy Induced Myelosuppression Treatment Market benefits from a robust pipeline of innovative therapies that offer new avenues for effective management. Development of targeted agents, such as CDK4/6 inhibitors and novel biologics, presents opportunities to improve patient outcomes and reduce chemotherapy-related complications. Emerging treatment modalities that focus on minimizing bone marrow suppression and enhancing hematopoietic recovery attract significant clinical and commercial interest. Continuous research and clinical trials foster advancements that could expand therapeutic options. It also encourages partnerships between pharmaceutical companies and research institutions to accelerate product development and approval processes.
Expansion of Personalized Medicine and Digital Health Integration
Personalized medicine and digital health integration create substantial growth opportunities within the U.S. Chemotherapy Induced Myelosuppression Treatment Market. Tailoring treatments based on genetic profiles and biomarker analysis enables more precise management of myelosuppression. Digital tools, including AI-driven predictive models and remote patient monitoring systems, improve treatment adherence and early detection of adverse effects. These technologies facilitate data-driven clinical decisions, enhancing patient care quality. The increasing adoption of personalized approaches and digital innovations supports market growth by improving therapeutic efficacy and patient engagement.
Market Segmentation Analysis
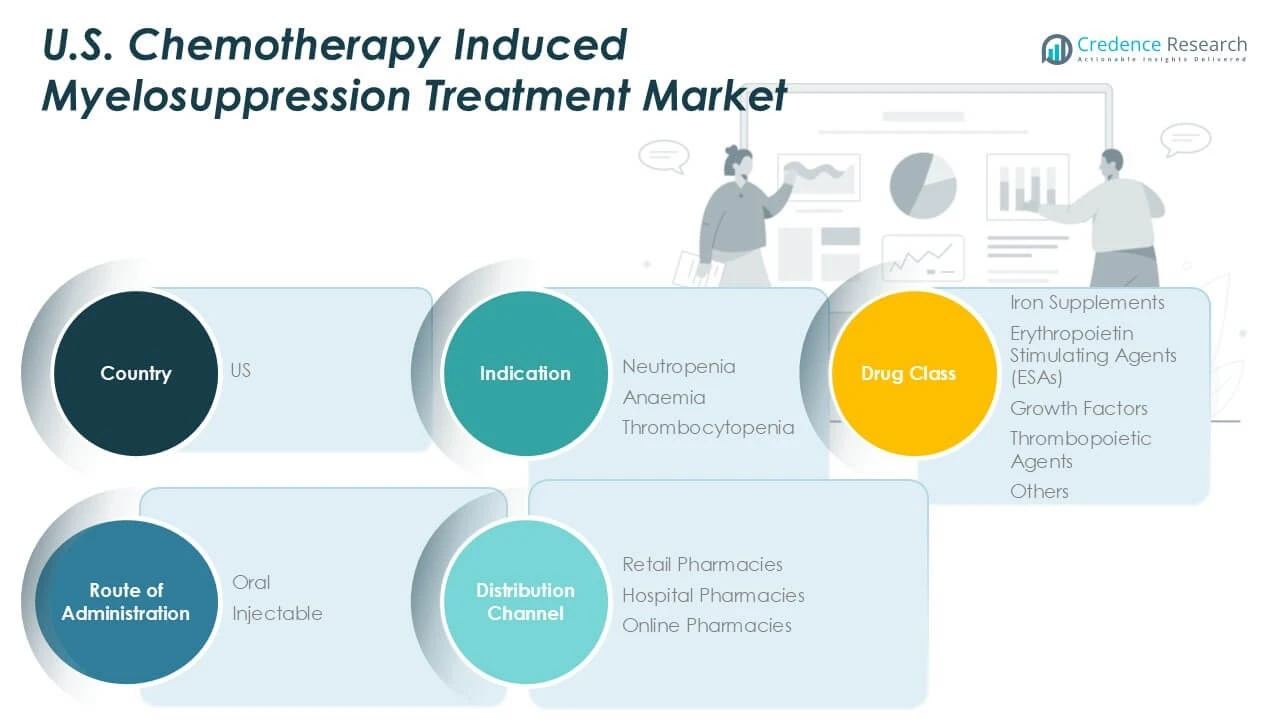
By indication
The market covers neutropenia, anemia, and thrombocytopenia, with neutropenia commanding the largest revenue share due to its high prevalence and associated infection risks. Treatments targeting anemia and thrombocytopenia also contribute significantly, reflecting the need for comprehensive supportive care during chemotherapy.
- For instance, thrombocytopenia is commonly observed in chemotherapy patients, increasing the risk of bleeding complications.
By drug class
The market includes iron supplements, erythropoietin stimulating agents (ESAs), growth factors, thrombopoietic agents, and others. Growth factors, such as granulocyte colony-stimulating factors (G-CSFs), dominate the market given their effectiveness in accelerating bone marrow recovery. ESAs and thrombopoietic agents hold substantial shares due to their roles in managing anemia and platelet deficiencies, respectively. Iron supplements form an essential adjunct in anemia management.
- For instance, IV iron supplementation has been shown to reduce ESA dosage requirements by up to 70%, improving anemia management.
By Route of Administration
The route of administration segments oral and injectable therapies, with injectable formulations leading due to rapid onset and higher efficacy in acute management. Oral therapies are gaining traction owing to patient convenience and improved compliance, especially in outpatient settings. Both routes remain critical in treatment protocols depending on clinical circumstances.
By Distribution Channel
Distribution channels comprise retail pharmacies, hospital pharmacies, and online pharmacies. Hospital pharmacies capture the majority market share, reflecting the administration of treatments within clinical settings. Retail pharmacies serve outpatient demands, while online pharmacies are expanding due to increasing digital adoption and patient preference for home delivery. Together, these segments define the comprehensive structure of the U.S. Chemotherapy Induced Myelosuppression Treatment Market.
Segments
Based on Indication
- Neutropenia
- Anaemia
- Thrombocytopenia
Based on Drug Class
- Iron Supplements
- Erythropoietin Stimulating Agents (ESAs)
- Growth Factors
- Thrombopoietic Agents
- Others
Based on Route of Administration
Based on Distribution Channel
- Retail Pharmacies
- Hospital Pharmacies
- Online Pharmacies
Regional Analysis
Northeast Chemotherapy Induced Myelosuppression Treatment Market
The Northeast region holds a significant share of the U.S. Chemotherapy Induced Myelosuppression Treatment Market, accounting for approximately 30% of the total market revenue. With a well-established healthcare infrastructure, leading hospitals, and cancer treatment centers, this region is a key player in the adoption of advanced therapies. The growing prevalence of cancer, combined with a high rate of chemotherapy treatments, contributes to its market dominance. Major pharmaceutical companies and research institutions based in the region drive the availability and development of innovative treatments for chemotherapy-induced myelosuppression. The region is expected to maintain its position, accounting for a substantial share of market revenue due to increasing awareness and early diagnosis of cancer.
Midwest Chemotherapy Induced Myelosuppression Treatment Market
The Midwest region represents a significant segment of the U.S. Chemotherapy Induced Myelosuppression Treatment Market, holding around 20% of the total market share. With expansive healthcare networks and growing patient populations, the demand for effective management of chemotherapy-induced myelosuppression remains high. Healthcare providers are increasingly adopting advanced supportive care therapies to improve patient outcomes. Market growth is supported by ongoing research and development activities in the region, focusing on enhancing treatment efficacy and availability. The Midwest region is expected to continue growing, capturing a notable market share in the coming years.
Southwest Chemotherapy Induced Myelosuppression Treatment Market
The Southwest region, including states like Texas and Arizona, exhibits a growing share of approximately 15% in the U.S. Chemotherapy Induced Myelosuppression Treatment Market. The region is home to a diverse population and a rising number of cancer diagnoses, particularly in the elderly population, which fuels the demand for effective treatment options. The expansion of specialized cancer treatment facilities in the Southwest, along with increasing healthcare access, drives the adoption of therapies for managing chemotherapy-induced myelosuppression. As the region invests in healthcare infrastructure, its market share is expected to increase steadily, contributing to the overall growth of the industry.
West Coast Chemotherapy Induced Myelosuppression Treatment Market
The West Coast holds a prominent market share of approximately 25% in the U.S. Chemotherapy Induced Myelosuppression Treatment Market. Known for its innovation-driven healthcare environment, the West Coast is a hub for cutting-edge treatments and clinical trials. The high concentration of biotechnology firms, research institutions, and hospitals specializing in cancer treatment fosters rapid adoption of new therapies. The region’s significant population, particularly in California, combined with an increasing focus on personalized medicine, supports the growth of chemotherapy-induced myelosuppression treatment options. The West Coast’s market share is expected to remain substantial, fueled by technological advancements and patient demand for advanced therapies.
Southeast Chemotherapy Induced Myelosuppression Treatment Market
The Southeast region is witnessing a steady rise in the U.S. Chemotherapy Induced Myelosuppression Treatment Market, holding a market share of around 10%. States like Florida and Georgia have seen growth in cancer cases, leading to increased chemotherapy treatments and the need for effective management of myelosuppression. The expansion of healthcare facilities and oncology centers, along with improved patient access to supportive care treatments, further boosts market growth. The Southeast region’s market share is expected to grow as healthcare providers increasingly incorporate new therapies into treatment protocols, improving overall patient care.
South Central Chemotherapy Induced Myelosuppression Treatment Market
The South Central region, covering states like Texas and Oklahoma, contributes significantly to the U.S. Chemotherapy Induced Myelosuppression Treatment Market, with an estimated market share of 10%. A rise in cancer incidence, combined with expanding healthcare access, particularly in rural areas, drives demand for effective treatments. Regional healthcare systems are increasingly adopting advanced therapies to manage chemotherapy-induced myelosuppression, further pushing the market forward. The South Central market share is projected to grow as healthcare infrastructure continues to improve, providing patients with greater access to innovative treatments and supportive care solutions.
Key players
- Novartis AG
- Amgen Inc.
- Dova Pharmaceuticals
- Mylan NV (Viatris)
- Walgreens Boots Alliance
- Pfizer Inc.
- Teva Pharmaceutical Industries Ltd.
- Johnson & Johnson
- CVS Health
Competitive Analysis
The U.S. Chemotherapy Induced Myelosuppression Treatment Market is highly competitive, with key players focusing on enhancing their product portfolios and expanding market presence. Companies like Novartis AG and Amgen Inc. lead the market with their advanced therapies, such as growth factors and erythropoiesis-stimulating agents, that help manage myelosuppression. Pfizer Inc. and Teva Pharmaceutical Industries Ltd. are also significant players, offering treatments for neutropenia and anemia management. Partnerships and collaborations are common among these companies to advance R\&D and ensure rapid commercialization of new therapies. The market’s growth is driven by innovation, strong distribution networks, and ongoing clinical trials aimed at improving patient outcomes. Companies are focusing on differentiated products, strategic pricing, and targeted marketing efforts to capture a larger share of the market.
Recent Developments
- In May 2025, Amgen presented findings at the American Society of Clinical Oncology (ASCO) meeting highlighting that its drug Nplate (romiplostim) was highly effective in preventing chemotherapy-induced thrombocytopenia (CIT). The pivotal trial demonstrated that 84% of patients treated with Nplate were able to continue full-dose chemotherapy without dose reductions, compared to only 36% in the placebo group. This marks a significant advancement, as there are currently no FDA-approved treatments for CIT.
- In October 2024, Walgreens Boots Alliance conducted a study evaluating the use of hematological oral oncolytics in cancer patients. The study demonstrated that patients who adhered to their oral cancer medications were more likely to have fewer hospital visits, shorter hospital stays, and lower overall medical costs, compared to those who did not adhere to their medication regimen.
- In April 2024, Teva Pharmaceutical Industries Ltd. and Alvotech announced that the U.S. Food and Drug Administration (FDA) approved SELARSDI (ustekinumab-aekn) injection for subcutaneous use, as a biosimilar to Stelara®, for the treatment of moderate to severe plaque psoriasis and for active psoriatic arthritis in adults and pediatric patients six years and older. SELARSDI is expected to launch in the U.S. in the first quarter of 2025.
- In June 2025, Johnson & Johnson showcased advancements in cancer innovation with more than 70 clinical and real-world studies at the American Society of Clinical Oncology (ASCO) and European Hematology Association (EHA) meetings. These studies highlighted the company’s commitment to transforming the science of genitourinary (GU) cancers.
Market Concentration and Characteristics
The U.S. Chemotherapy Induced Myelosuppression Treatment Market exhibits moderate to high concentration, dominated by a few key players such as Novartis AG, Amgen Inc., and Pfizer Inc. These companies lead with their advanced therapeutic portfolios, including growth factors, erythropoiesis-stimulating agents, and thrombopoietic agents. The market is characterized by continuous innovation and strategic partnerships, with players investing heavily in R&D to develop more effective and targeted treatments. The increasing prevalence of cancer and rising demand for supportive care therapies further intensify competition. Market players focus on expanding their product offerings, improving treatment efficacy, and gaining regulatory approvals to maintain a competitive edge. With a strong emphasis on personalized medicine and technological advancements, it is expected that competition will continue to grow, making market dynamics increasingly complex and fostering ongoing product differentiation.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Report Coverage
The research report offers an in-depth analysis based on Indication, Drug Class, Route of Administration, Distribution Channel and Region. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The U.S. Chemotherapy Induced Myelosuppression Treatment Market is expected to see sustained growth driven by increasing cancer diagnoses and a higher number of chemotherapy treatments.
- Targeted therapies focusing on specific myelosuppression pathways will become more prominent, improving treatment efficacy and reducing side effects.
- The continued shift towards personalized medicine will lead to more tailored treatments, optimizing patient outcomes and reducing unnecessary complications.
- With the growing adoption of biosimilars, cost-effective alternatives to biologic therapies will become increasingly available, expanding access to treatments.
- Digital health technologies, including AI-driven predictive models and remote monitoring, will enhance treatment adherence and improve early intervention for myelosuppression.
- As neutropenia remains a major challenge in chemotherapy, new therapies aimed at preventing or managing neutropenia will drive market growth.
- Accelerated regulatory pathways and the approval of new treatment options will help bring innovative therapies to market more quickly, supporting overall market expansion.
- The demand for oral therapies, providing convenience and improving patient compliance, will rise, shifting the treatment landscape.
- Strategic partnerships between pharmaceutical companies and research institutions will continue to drive innovation and accelerate the commercialization of new treatments.
- Enhanced patient education and awareness about chemotherapy-induced myelosuppression will result in more patients seeking timely and effective treatment, expanding the overall market.





