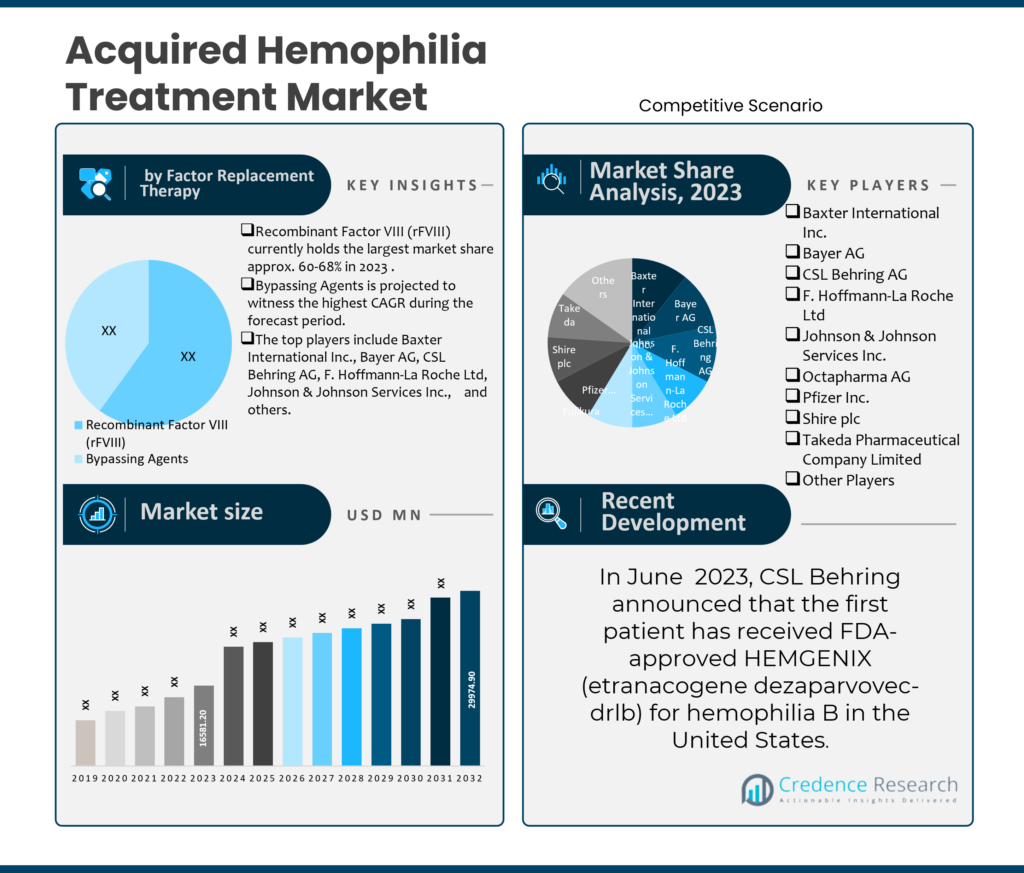
1. Preface
1.1. Report Description
1.1.1. Purpose of the Report
1.1.2. Target Audience
1.1.3. USP and Key Offerings
1.2. Research Scope
1.3. Market Introduction
2. Executive Summary
2.1. Market Snapshot: Global Acquired Hemophilia Treatment Market
2.1.1. Global Acquired Hemophilia Treatment Market, By Factor Replacement Therapy
2.1.2. Global Acquired Hemophilia Treatment Market, By Immunosuppressive Therapy
2.1.3. Global Acquired Hemophilia Treatment Market, By Plasmapheresis and Immunoadsorption
2.1.4. Global Acquired Hemophilia Treatment Market, By Supportive Care
2.1.5. Global Acquired Hemophilia Treatment Market, By Treatment Setting
2.1.6. Global Acquired Hemophilia Treatment Market, By Region
2.2. Insights from Primary Respondents
3. Market Dynamics & Factors Analysis
3.1. Introduction
3.1.1. Global Acquired Hemophilia Treatment Market Value, 2019-2032, (US$ Mn)
3.1.2. Y-o-Y Growth Trend Analysis
3.2. Market Dynamics
3.2.1. Acquired Hemophilia Treatment Drivers
3.2.2. Acquired Hemophilia Treatment Market Restraints
3.2.3. Acquired Hemophilia Treatment Market Opportunities
3.2.4. Major Acquired Hemophilia Treatment Industry Challenges
3.3. Growth and Development Patterns
3.4. Investment Feasibility Analysis
3.5. Market Opportunity Analysis
3.5.1. Factor Replacement Therapy
3.5.2. Immunosuppressive Therapy
3.5.3. Plasmapheresis and Immunoadsorption
3.5.4. Supportive Care
3.5.5. Treatment Setting
3.5.6. Geography
4. Market Competitive Landscape Analysis
4.1. Company Market Share Analysis, 2023
4.1.1. Global Acquired Hemophilia Treatment Market: Company Market Share, Value 2023
4.1.2. Global Acquired Hemophilia Treatment Market: Top 6 Company Market Share, Value 2023
4.1.3. Global Acquired Hemophilia Treatment Market: Top 3 Company Market Share, Value 2023
4.2. Global Acquired Hemophilia Treatment Market: Company Revenue Share Analysis, 2023
4.3. Company Assessment Metrics, 2023
4.3.1. Stars
4.3.2. Emerging Leaders
4.3.3. Pervasive Players
4.3.4. Participants
4.4. Startups/ SMEs Assessment Metrics, 2023
4.4.1. Progressive Companies
4.4.2. Responsive Companies
4.4.3. Dynamic Companies
4.4.4. Starting Blocks
4.5. Strategic Development
4.5.1. Acquisition and Mergers
4.5.2. New Product Launch
4.5.3. Regional Expansion
4.5.4. Partnerships
4.6. Key Player Product Matrix
4.7. Potential for New Players in the Global Acquired Hemophilia Treatment Market
5. Premium Insights
5.1. STAR (Situation, Task, Action, Results) Analysis
5.2. Porter’s Five Forces Analysis
5.2.1. Threat of New Entrants
5.2.2. Bargaining Power of Buyers/Consumers
5.2.3. Bargaining Power of Suppliers
5.2.4. Threat of Substitute Types
5.2.5. Intensity of Competitive Rivalry
5.3. PESTEL Analysis
5.3.1. Political Factors
5.3.2. Economic Factors
5.3.3. Social Factors
5.3.4. Technological Factors
5.3.5. Environmental Factors
5.3.6. Legal Factors
5.4. Key Market Trends
5.4.1. Demand Side Trends
5.4.2. Supply Side Trends
5.5. Value Chain Analysis
5.6. Technology Analysis
5.6.1. Research and development in the global market
5.6.2. Patent Analysis
5.6.3. Emerging technologies and their potential disruption to the market
5.7. Consumer Behaviour Analysis
5.7.1. Consumer Preferences and Expectations
5.7.2. Factors Influencing Consumer Buying Decisions
5.7.2.1. North America
5.7.2.2. Europe
5.7.2.3. Asia Pacific
5.7.2.4. Latin America
5.7.2.5. Middle East and Africa
5.7.3. Consumer Pain Points
5.8. Analysis and Recommendations
5.9. Adjacent Market Analysis
6. Market Positioning of Key Players, 2023
6.1. Company market share of key players, 2023
6.2. Competitive Benchmarking
6.3. Market Positioning of Key Vendors
6.4. Geographical Presence Analysis
6.5. Major Strategies Adopted by Key Players
6.5.1. Key Strategies Analysis
6.5.2. Mergers and Acquisitions
6.5.3. Partnerships
6.5.4. Product Launch
6.5.5. Geographical Expansion
6.5.6. Others
7. Impact Analysis of COVID 19 and Russia – Ukraine War on Acquired Hemophilia Treatment Market
7.1. Ukraine-Russia War Impact
7.1.1. Uncertainty and Economic Instability
7.1.2. Supply chain disruptions
7.1.3. Regional market shifts
7.1.4. Shift in government priorities
7.2. COVID-19 Impact Analysis
7.2.1. Supply Chain Disruptions
7.2.2. Demand Fluctuations
7.2.3. Shift in Product Mix
7.2.4. Reduced Industrial Activity
7.2.5. Regional Impact Analysis
7.2.5.1. North America
7.2.5.2. Europe
7.2.5.3. Asia Pacific
7.2.5.4. Latin America
7.2.5.5. Middle East and Africa
8. Global Acquired Hemophilia Treatment Market, By Factor Replacement Therapy
8.1. Global Acquired Hemophilia Treatment Market Overview, by Factor Replacement Therapy
8.1.1. Global Acquired Hemophilia Treatment Market Revenue Share, By Factor Replacement Therapy, 2023 Vs 2032 (in %)
8.2. Recombinant Factor VIII (rFVIII)
8.2.1. Global Acquired Hemophilia Treatment Market, By Recombinant Factor VIII (rFVIII), By Region, 2019-2032(US$ Mn)
8.2.2. Market Dynamics for Recombinant Factor VIII (rFVIII)
8.2.2.1. Drivers
8.2.2.2. Restraints
8.2.2.3. Opportunities
8.2.2.4. Trends
8.3. Bypassing Agents
8.3.1. Global Acquired Hemophilia Treatment Market, By Bypassing Agents, By Region, 2019-2032 (US$ Mn)
8.3.2. Market Dynamics for Bypassing Agents
8.3.2.1. Drivers
8.3.2.2. Restraints
8.3.2.3. Opportunities
8.3.2.4. Trends
9. Global Acquired Hemophilia Treatment Market, By Immunosuppressive Therapy
9.1. Global Acquired Hemophilia Treatment Market Overview, by Immunosuppressive Therapy
9.1.1. Global Acquired Hemophilia Treatment Market Revenue Share, By Immunosuppressive Therapy, 2023 Vs 2032 (in %)
9.2. Corticosteroids
9.2.1. Global Acquired Hemophilia Treatment Market, By Corticosteroids, By Region, 2019-2032 (US$ Mn)
9.2.2. Market Dynamics for Corticosteroids
9.2.2.1. Drivers
9.2.2.2. Restraints
9.2.2.3. Opportunities
9.2.2.4. Trends
9.3. Immunosuppressants
9.3.1. Global Acquired Hemophilia Treatment Market, By Immunosuppressants, By Region, 2019-2032 (US$ Mn)
9.3.2. Market Dynamics for Immunosuppressants
9.3.2.1. Drivers
9.3.2.2. Restraints
9.3.2.3. Opportunities
9.3.2.4. Trends
10. Global Acquired Hemophilia Treatment Market, By Plasmapheresis and Immunoadsorption
10.1. Global Acquired Hemophilia Treatment Market Overview, by Plasmapheresis and Immunoadsorption
10.1.1. Global Acquired Hemophilia Treatment Market Revenue Share, By Plasmapheresis and Immunoadsorption, 2023 Vs 2032 (in %)
10.2. Plasmapheresis
10.2.1. Global Acquired Hemophilia Treatment Market, By Plasmapheresis, By Region, 2019-2032 (US$ Mn)
10.2.2. Market Dynamics for Plasmapheresis
10.2.2.1. Drivers
10.2.2.2. Restraints
10.2.2.3. Opportunities
10.2.2.4. Trends
10.3. Immunoadsorption
10.3.1. Global Acquired Hemophilia Treatment Market, By Immunoadsorption, By Region, 2019-2032 (US$ Mn)
10.3.2. Market Dynamics for Immunoadsorption
10.3.2.1. Drivers
10.3.2.2. Restraints
10.3.2.3. Opportunities
10.3.2.4. Trends
11. Global Acquired Hemophilia Treatment Market, By Supportive Care
11.1. Global Acquired Hemophilia Treatment Market Overview, by Supportive Care
11.1.1. Global Acquired Hemophilia Treatment Market Revenue Share, By Supportive Care, 2023 Vs 2032 (in %)
11.2. Hemostatic Agents
11.2.1. Global Acquired Hemophilia Treatment Market, By Hemostatic Agents, By Region, 2019-2032 (US$ Mn)
11.2.2. Market Dynamics for Hemostatic Agents
11.2.2.1. Drivers
11.2.2.2. Restraints
11.2.2.3. Opportunities
11.2.2.4. Trends
11.3. Symptomatic Treatment
11.3.1. Global Acquired Hemophilia Treatment Market, By Symptomatic Treatment, By Region, 2019-2032 (US$ Mn)
11.3.2. Market Dynamics for Symptomatic Treatment
11.3.2.1. Drivers
11.3.2.2. Restraints
11.3.2.3. Opportunities
11.3.2.4. Trends
12. Global Acquired Hemophilia Treatment Market, By Treatment Setting
12.1. Global Acquired Hemophilia Treatment Market Overview, by Treatment Setting
12.1.1. Global Acquired Hemophilia Treatment Market Revenue Share, By Treatment Setting, 2023 Vs 2032 (in %)
12.2. Hospital-based Treatment
12.2.1. Global Acquired Hemophilia Treatment Market, By Hospital-based Treatment, By Region, 2019-2032 (US$ Mn)
12.2.2. Market Dynamics for Hospital-based Treatment
12.2.2.1. Drivers
12.2.2.2. Restraints
12.2.2.3. Opportunities
12.2.2.4. Trends
12.3. Outpatient Treatment
12.3.1. Global Acquired Hemophilia Treatment Market, By Outpatient Treatment, By Region, 2019-2032 (US$ Mn)
12.3.2. Market Dynamics for Outpatient Treatment
12.3.2.1. Drivers
12.3.2.2. Restraints
12.3.2.3. Opportunities
12.3.2.4. Trends
13. Global Acquired Hemophilia Treatment Market, By Region
13.1. Global Acquired Hemophilia Treatment Market Overview, by Region
13.1.1. Global Acquired Hemophilia Treatment Market, By Region, 2023 vs 2032 (in%)
13.2. Factor Replacement Therapy
13.2.1. Global Acquired Hemophilia Treatment Market, By Factor Replacement Therapy, 2019-2032 (US$ Mn)
13.3. Immunosuppressive Therapy
13.3.1. Global Acquired Hemophilia Treatment Market, By Immunosuppressive Therapy, 2019-2032 (US$ Mn)
13.4. Plasmapheresis and Immunoadsorption
13.4.1. Global Acquired Hemophilia Treatment Market, By Plasmapheresis and Immunoadsorption, 2019-2032 (US$ Mn)
13.5. Supportive Care
13.5.1. Global Acquired Hemophilia Treatment Market, By Supportive Care, 2019-2032 (US$ Mn)
13.6. Treatment Setting
13.6.1. Global Acquired Hemophilia Treatment Market, By Treatment Setting, 2019-2032 (US$ Mn)
14. North America Acquired Hemophilia Treatment Market Analysis
14.1. Overview
14.1.1. Market Dynamics for North America
14.1.1.1. Drivers
14.1.1.2. Restraints
14.1.1.3. Opportunities
14.1.1.4. Trends
14.2. North America Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2032(US$ Mn)
14.2.1. Overview
14.2.2. SRC Analysis
14.3. North America Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2032(US$ Mn)
14.3.1. Overview
14.3.2. SRC Analysis
14.4. North America Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2032(US$ Mn)
14.4.1. Overview
14.4.2. SRC Analysis
14.5. North America Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2032(US$ Mn)
14.5.1. Overview
14.5.2. SRC Analysis
14.6. North America Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2032(US$ Mn)
14.6.1. Overview
14.6.2. SRC Analysis
14.7. North America Acquired Hemophilia Treatment Market, by Country, 2019-2032(US$ Mn)
14.7.1. North America Acquired Hemophilia Treatment Market, by Country, 2023 Vs 2032 (in%)
14.7.2. U.S.
14.7.3. Canada
14.7.4. Mexico
15. Europe Acquired Hemophilia Treatment Market Analysis
15.1. Overview
15.1.1. Market Dynamics for North America
15.1.1.1. Drivers
15.1.1.2. Restraints
15.1.1.3. Opportunities
15.1.1.4. Trends
15.2. Europe Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2032(US$ Mn)
15.2.1. Overview
15.2.2. SRC Analysis
15.3. Europe Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2032(US$ Mn)
15.3.1. Overview
15.3.2. SRC Analysis
15.4. Europe Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2032(US$ Mn)
15.4.1. Overview
15.4.2. SRC Analysis
15.5. Europe Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2032(US$ Mn)
15.5.1. Overview
15.5.2. SRC Analysis
15.6. Europe Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2032(US$ Mn)
15.6.1. Overview
15.6.2. SRC Analysis
15.7. Europe Acquired Hemophilia Treatment Market, by Country, 2019-2032 (US$ Mn)
15.7.1. Europe Acquired Hemophilia Treatment Market, by Country, 2023 Vs 2032 (in%)
15.7.2. UK
15.7.3. France
15.7.4. Germany
15.7.5. Italy
15.7.6. Spain
15.7.7. Benelux
15.7.8. Russia
15.7.9. Rest of Europe
16. Asia Pacific Acquired Hemophilia Treatment Market Analysis
16.1. Overview
16.1.1. Market Dynamics for North America
16.1.1.1. Drivers
16.1.1.2. Restraints
16.1.1.3. Opportunities
16.1.1.4. Trends
16.2. Asia Pacific Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2032(US$ Mn)
16.2.1. Overview
16.2.2. SRC Analysis
16.3. Asia Pacific Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2032(US$ Mn)
16.3.1. Overview
16.3.2. SRC Analysis
16.4. Asia Pacific Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2032(US$ Mn)
16.4.1. Overview
16.4.2. SRC Analysis
16.5. Asia Pacific Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2032(US$ Mn)
16.5.1. Overview
16.5.2. SRC Analysis
16.6. Asia Pacific Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2032(US$ Mn)
16.6.1. Overview
16.6.2. SRC Analysis
16.7. Asia Pacific Acquired Hemophilia Treatment Market, by Country, 2019-2032 (US$ Mn)
16.7.1. Asia Pacific Acquired Hemophilia Treatment Market, by Country, 2023 Vs 2032 (in%)
16.7.2. China
16.7.3. Japan
16.7.4. India
16.7.5. South Korea
16.7.6. South East Asia
16.7.7. Rest of Asia Pacific
17. Latin America Acquired Hemophilia Treatment Market Analysis
17.1. Overview
17.1.1. Market Dynamics for North America
17.1.1.1. Drivers
17.1.1.2. Restraints
17.1.1.3. Opportunities
17.1.1.4. Trends
17.2. Latin America Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2032(US$ Mn)
17.2.1. Overview
17.2.2. SRC Analysis
17.3. Latin America Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2032(US$ Mn)
17.3.1. Overview
17.3.2. SRC Analysis
17.4. Latin America Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2032(US$ Mn)
17.4.1. Overview
17.4.2. SRC Analysis
17.5. Latin America Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2032(US$ Mn)
17.5.1. Overview
17.5.2. SRC Analysis
17.6. Latin America Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2032(US$ Mn)
17.6.1. Overview
17.6.2. SRC Analysis
17.7. Latin America Acquired Hemophilia Treatment Market, by Country, 2019-2032 (US$ Mn)
17.7.1. Latin America Acquired Hemophilia Treatment Market, by Country, 2023 Vs 2032 (in%)
17.7.2. Brazil
17.7.3. Argentina
17.7.4. Rest of Latin America
18. Middle East Acquired Hemophilia Treatment Market Analysis
18.1. Overview
18.1.1. Market Dynamics for North America
18.1.1.1. Drivers
18.1.1.2. Restraints
18.1.1.3. Opportunities
18.1.1.4. Trends
18.2. Middle East Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2032(US$ Mn)
18.2.1. Overview
18.2.2. SRC Analysis
18.3. Middle East Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2032(US$ Mn)
18.3.1. Overview
18.3.2. SRC Analysis
18.4. Middle East Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2032(US$ Mn)
18.4.1. Overview
18.4.2. SRC Analysis
18.5. Middle East Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2032(US$ Mn)
18.5.1. Overview
18.5.2. SRC Analysis
18.6. Middle East Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2032(US$ Mn)
18.6.1. Overview
18.6.2. SRC Analysis
18.7. Middle East Acquired Hemophilia Treatment Market, by Country, 2019-2032 (US$ Mn)
18.7.1. Middle East Acquired Hemophilia Treatment Market, by Country, 2023 Vs 2032 (in%)
18.7.2. UAE
18.7.3. Saudi Arabia
18.7.4. Rest of Middle East
19. Africa Acquired Hemophilia Treatment Market Analysis
19.1. Overview
19.1.1. Market Dynamics for North America
19.1.1.1. Drivers
19.1.1.2. Restraints
19.1.1.3. Opportunities
19.1.1.4. Trends
19.2. Africa Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2032(US$ Mn)
19.2.1. Overview
19.2.2. SRC Analysis
19.3. Africa Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2032(US$ Mn)
19.3.1. Overview
19.3.2. SRC Analysis
19.4. Africa Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2032(US$ Mn)
19.4.1. Overview
19.4.2. SRC Analysis
19.5. Africa Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2032(US$ Mn)
19.5.1. Overview
19.5.2. SRC Analysis
19.6. Africa Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2032(US$ Mn)
19.6.1. Overview
19.6.2. SRC Analysis
19.7. Africa Acquired Hemophilia Treatment Market, by Country, 2019-2032 (US$ Mn)
19.7.1. Middle East Acquired Hemophilia Treatment Market, by Country, 2023 Vs 2032 (in%)
19.7.2. South Africa
19.7.3. Egypt
19.7.4. Rest of Africa
20. Company Profiles
20.1. Baxter International Inc.
20.1.1. Company Overview
20.1.2. Products/Services Portfolio
20.1.3. Geographical Presence
20.1.4. SWOT Analysis
20.1.5. Financial Summary
20.1.5.1. Market Revenue and Net Profit (2019-2023)
20.1.5.2. Business Segment Revenue Analysis
20.1.5.3. Geographical Revenue Analysis
20.2. Bayer AG
20.3. CSL Behring AG
20.4. F. Hoffmann-La Roche Ltd
20.5. Johnson & Johnson Services Inc.
20.6. Octapharma AG
20.7. Pfizer Inc.
20.8. Shire plc
20.9. Takeda Pharmaceutical Company Limited
20.10. Others
21. Research Methodology
21.1. Research Methodology
21.2. Phase I – Secondary Research
21.3. Phase II – Data Modelling
21.3.1. Company Share Analysis Model
21.3.2. Revenue Based Modelling
21.4. Phase III – Primary Research
21.5. Research Limitations
21.5.1. Assumptions
List of Figures
FIG. 1 Global Acquired Hemophilia Treatment Market: Research Methodology
FIG. 2 Market Size Estimation – Top Down & Bottom up Approach
FIG. 3 Global Acquired Hemophilia Treatment Market Segmentation
FIG. 4 Global Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2023 (US$ Mn)
FIG. 5 Global Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2023 (US$ Mn)
FIG. 6 Global Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2023 (US$ Mn)
FIG. 7 Global Acquired Hemophilia Treatment Market, by Supportive Care, 2023 (US$ Mn)
FIG. 8 Global Acquired Hemophilia Treatment Market, by Treatment Setting, 2023 (US$ Mn)
FIG. 9 Global Acquired Hemophilia Treatment Market, by Geography, 2023 (US$ Mn)
FIG. 10 Attractive Investment Proposition, by Factor Replacement Therapy, 2023
FIG. 11 Attractive Investment Proposition, by Immunosuppressive Therapy, 2023
FIG. 12 Attractive Investment Proposition, by Plasmapheresis and Immunoadsorption, 2023
FIG. 13 Attractive Investment Proposition, by Supportive Care, 2023
FIG. 14 Attractive Investment Proposition, by Treatment Setting, 2023
FIG. 15 Attractive Investment Proposition, by Geography, 2023
FIG. 16 Global Market Share Analysis of Key Acquired Hemophilia Treatment Market Manufacturers, 2023
FIG. 17 Global Market Positioning of Key Acquired Hemophilia Treatment Market Manufacturers, 2023
FIG. 18 Global Acquired Hemophilia Treatment Market Value Contribution, By Factor Replacement Therapy, 2023&2032 (Value %)
FIG. 19 Global Acquired Hemophilia Treatment Market, by Recombinant Factor VIII (rFVIII), By Region, 2019-2023 (US$ Mn)
FIG. 20 Global Acquired Hemophilia Treatment Market, by Bypassing Agents, By Region, 2019-2023 (US$ Mn)
FIG. 21 Global Acquired Hemophilia Treatment Market Value Contribution, By Immunosuppressive Therapy, 2023&2032 (Value %)
FIG. 22 Global Acquired Hemophilia Treatment Market, by Corticosteroids, By Region, 2019-2023 (US$ Mn)
FIG. 23 Global Acquired Hemophilia Treatment Market, by Immunosuppressants, By Region, 2019-2023 (US$ Mn)
FIG. 24 Global Acquired Hemophilia Treatment Market Value Contribution, By Plasmapheresis and Immunoadsorption, 2023&2032 (Value %)
FIG. 25 Global Acquired Hemophilia Treatment Market, by Plasmapheresis, By Region, 2019-2023 (US$ Mn)
FIG. 26 Global Acquired Hemophilia Treatment Market, by Immunoadsorption, By Region, 2019-2023 (US$ Mn)
FIG. 27 Global Acquired Hemophilia Treatment Market Value Contribution, By Supportive Care, 2023&2032 (Value %)
FIG. 28 Global Acquired Hemophilia Treatment Market, by Hemostatic Agents, By Region, 2019-2023 (US$ Mn)
FIG. 29 Global Acquired Hemophilia Treatment Market, by Symptomatic Treatment, By Region, 2019-2023 (US$ Mn)
FIG. 30 Global Acquired Hemophilia Treatment Market Value Contribution, By Treatment Setting, 2023&2032 (Value %)
FIG. 31 Global Acquired Hemophilia Treatment Market, by Hospital-based Treatment, By Region, 2019-2023 (US$ Mn)
FIG. 32 Global Acquired Hemophilia Treatment Market, by Outpatient Treatment, By Region, 2019-2023 (US$ Mn)
FIG. 33 North America Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 34 U.S. Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 35 Canada Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 36 Mexico Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 37 Europe Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 38 Germany Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 39 France Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 40 U.K. Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 41 Italy Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 42 Spain Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 43 Benelux Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 44 Russia Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 45 Rest of Europe Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 46 Asia Pacific Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 47 China Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 48 Japan Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 49 India Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 50 South Korea Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 51 South-East Asia Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 52 Rest of Asia Pacific Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 53 Latin America Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 54 Brazil Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 55 Argentina Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 56 Rest of Latin America Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 57 Middle East Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 58 UAE Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 59 Saudi Arabia Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 60 Rest of Middle East Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 61 Africa Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 62 South Africa Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 63 Egypt Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
FIG. 64 Rest of Africa Acquired Hemophilia Treatment Market, 2019-2032 (US$ Mn)
List of Tables
TABLE 1 Market Snapshot: Global Acquired Hemophilia Treatment Market
TABLE 2 Global Acquired Hemophilia Treatment Market: Market Drivers Impact Analysis
TABLE 3 Global Acquired Hemophilia Treatment Market: Market Restraints Impact Analysis
TABLE 4 Global Acquired Hemophilia Treatment Market, by Competitive Benchmarking, 2023
TABLE 5 Global Acquired Hemophilia Treatment Market, by Geographical Presence Analysis, 2023
TABLE 6 Global Acquired Hemophilia Treatment Market, by Key Strategies Analysis, 2023
TABLE 7 Global Acquired Hemophilia Treatment Market, by Recombinant Factor VIII (rFVIII), By Region, 2019-2023 (US$ Mn)
TABLE 8 Global Acquired Hemophilia Treatment Market, by Recombinant Factor VIII (rFVIII), By Region, 2024-2032 (US$ Mn)
TABLE 9 Global Acquired Hemophilia Treatment Market, by Bypassing Agents, By Region, 2019-2023 (US$ Mn)
TABLE 10 Global Acquired Hemophilia Treatment Market, by Bypassing Agents, By Region, 2019-2023 (US$ Mn)
TABLE 11 Global Acquired Hemophilia Treatment Market, by Corticosteroids, By Region, 2019-2023 (US$ Mn)
TABLE 12 Global Acquired Hemophilia Treatment Market, by Corticosteroids, By Region, 2019-2023 (US$ Mn)
TABLE 13 Global Acquired Hemophilia Treatment Market, by Immunosuppressants, By Region, 2019-2023 (US$ Mn)
TABLE 14 Global Acquired Hemophilia Treatment Market, by Immunosuppressants, By Region, 2019-2023 (US$ Mn)
TABLE 15 Global Acquired Hemophilia Treatment Market, by Plasmapheresis, By Region, 2019-2023 (US$ Mn)
TABLE 16 Global Acquired Hemophilia Treatment Market, by Plasmapheresis, By Region, 2019-2023 (US$ Mn)
TABLE 17 Global Acquired Hemophilia Treatment Market, by Immunoadsorption, By Region, 2019-2023 (US$ Mn)
TABLE 18 Global Acquired Hemophilia Treatment Market, by Immunoadsorption, By Region, 2019-2023 (US$ Mn)
TABLE 19 Global Acquired Hemophilia Treatment Market, by Hemostatic Agents, By Region, 2019-2023 (US$ Mn)
TABLE 20 Global Acquired Hemophilia Treatment Market, by Hemostatic Agents, By Region, 2019-2023 (US$ Mn)
TABLE 21 Global Acquired Hemophilia Treatment Market, by Symptomatic Treatment, By Region, 2019-2023 (US$ Mn)
TABLE 22 Global Acquired Hemophilia Treatment Market, by Symptomatic Treatment, By Region, 2019-2023 (US$ Mn)
TABLE 23 Global Acquired Hemophilia Treatment Market, by Hospital-based Treatment, By Region, 2019-2023 (US$ Mn)
TABLE 24 Global Acquired Hemophilia Treatment Market, by Hospital-based Treatment, By Region, 2019-2023 (US$ Mn)
TABLE 25 Global Acquired Hemophilia Treatment Market, by Outpatient Treatment, By Region, 2019-2023 (US$ Mn)
TABLE 26 Global Acquired Hemophilia Treatment Market, by Outpatient Treatment, By Region, 2019-2023 (US$ Mn)
TABLE 27 Region, 2019-2023 (US$ Mn)
TABLE 28 Global Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 29 Global Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 30 Global Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 31 Global Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 32 Global Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 33 Global Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 34 Global Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 35 Global Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 36 Global Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 37 Global Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 38 Global Acquired Hemophilia Treatment Market, by Region, 2019-2023 (US$ Mn)
TABLE 39 Global Acquired Hemophilia Treatment Market, by Region, 2024-2032 (US$ Mn)
TABLE 40 North America Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 41 North America Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 42 North America Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 43 North America Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 44 North America Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 45 North America Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 46 North America Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 47 North America Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 48 North America Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 49 North America Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 50 North America Acquired Hemophilia Treatment Market, by Country, 2019-2023 (US$ Mn)
TABLE 51 North America Acquired Hemophilia Treatment Market, by Country, 2024-2032 (US$ Mn)
TABLE 52 United States Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 53 United States Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 54 United States Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 55 United States Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 56 United States Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 57 United States Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 58 United States Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 59 United States Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 60 United States Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 61 United States Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 62 Canada Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 63 Canada Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 64 Canada Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 65 Canada Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 66 Canada Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 67 Canada Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 68 Canada Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 69 Canada Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 70 Canada ca Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 71 Canada Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 72 Mexico Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 73 Mexico Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 74 Mexico Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 75 Mexico Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 76 Mexico Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 77 Mexico Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 78 Mexico Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 79 Mexico Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 80 Mexico Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 81 Mexico Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 82 Europe Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 83 Europe Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 84 Europe Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 85 Europe Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 86 Europe Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 87 Europe Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 88 Europe Acquired Hemophilia Treatment Market, by Country, 2019-2023 (US$ Mn)
TABLE 89 Europe Acquired Hemophilia Treatment Market, by Country, 2024-2032 (US$ Mn)
TABLE 90 Europe Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 91 Europe Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 92 Europe Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 93 Europe Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 94 Germany Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 95 Germany Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 96 Germany Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 97 Germany Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 98 Germany Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 99 Germany Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 100 Germany Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 101 Germany Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 102 Germany Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 103 France Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 104 France Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 105 France Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 106 France Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 107 France Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 108 France Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 109 France Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 110 France Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 111 France Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 112 France Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 113 United Kingdom Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 114 United Kingdom Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 115 United Kingdom Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 116 United Kingdom Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 117 United Kingdom Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 118 United Kingdom Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 119 United Kingdom Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 120 United Kingdom Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 121 United Kingdom Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 122 United Kingdom Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 123 Italy Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 124 Italy Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 125 Italy Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 126 Italy Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 127 Italy Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 128 Italy Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 129 Italy Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 130 Italy Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 131 Italy Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 132 Italy Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 133 Spain Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 134 Spain Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 135 Spain Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 136 Spain Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 137 Spain Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 138 Spain Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 139 Spain Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 140 Spain Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 141 Spain Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 142 Spain Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 143 Benelux Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 144 Benelux Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 145 Benelux Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 146 Benelux Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 147 Benelux Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 148 Benelux Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 149 Benelux Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 150 Benelux Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 151 Benelux Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 152 Benelux Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 153 Russia Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 154 Russia Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 155 Russia Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 156 Russia Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 157 Russia Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 158 Russia Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 159 Russia Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 160 Russia Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 161 Russia Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 162 Russia Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 163 Rest of Europe Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 164 Rest of Europe Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 165 Rest of Europe Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 166 Rest of Europe Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 167 Rest of Europe Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 168 Rest of Europe Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 169 Rest of Europe Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 170 Rest of Europe Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 171 Rest of Europe Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 172 Rest of Europe Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 173 Asia Pacific Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 174 Asia Pacific Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 175 Asia Pacific Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 176 Asia Pacific Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 177 Asia Pacific Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 178 Asia Pacific Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 179 Asia Pacific Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 180 Asia Pacific Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 181 Asia Pacific Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 182 Asia Pacific Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 183 China Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 184 China Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 185 China Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 186 China Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 187 China Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 188 China Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 189 China Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 190 China Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 191 China Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 192 China Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 193 Japan Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 194 Japan Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 195 Japan Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 196 Japan Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 197 Japan Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 198 Japan Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 199 Japan Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 200 Japan Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 201 Japan Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 202 Japan Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 203 India Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 204 India Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 205 India Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 206 India Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 207 India Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 208 India Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 209 India Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 210 India Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 211 India Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 212 India Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 213 South Korea Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 214 South Korea Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 215 South Korea Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 216 South Korea Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 217 South Korea Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 218 South Korea Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 219 South Korea Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 220 South Korea Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 221 South Korea Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 222 South Korea Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 223 South-East Asia Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 224 South-East Asia Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 225 South-East Asia Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 226 South-East Asia Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 227 South-East Asia Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 228 South-East Asia Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 229 South-East Asia Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 230 South-East Asia Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 231 South-East Asia Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 232 South-East Asia Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 233 Rest of Asia Pacific Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 234 Rest of Asia Pacific Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 235 Rest of Asia Pacific Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 236 Rest of Asia Pacific Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 237 Rest of Asia Pacific Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 238 Rest of Asia Pacific Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 239 Rest of Asia Pacific Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 240 Rest of Asia Pacific Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 241 Rest of Asia Pacific Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 242 Rest of Asia Pacific Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 243 Latin America Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 244 Latin America Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 245 Latin America Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 246 Latin America Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 247 Latin America Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 248 Latin America Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 249 Latin America Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 250 Latin America Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 251 Latin America Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 252 Latin America Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 253 Brazil Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 254 Brazil Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 255 Brazil Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 256 Brazil Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 257 Brazil Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 258 Brazil Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 259 Brazil Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 260 Brazil Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 261 Brazil Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 262 Brazil Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 263 Argentina Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 264 Argentina Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 265 Argentina Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 266 Argentina Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 267 Argentina Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 268 Argentina Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 269 Argentina Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 270 Argentina Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 271 Argentina Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 272 Argentina Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 273 Rest of Latin America Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 274 Rest of Latin America Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 275 Rest of Latin America Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 276 Rest of Latin America Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 277 Rest of Latin America Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 278 Rest of Latin America Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 279 Rest of Latin America Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 280 Rest of Latin America Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 281 Rest of Latin America Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 282 Rest of Latin America Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 283 Middle East Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 284 Middle East Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 285 Middle East Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 286 Middle East Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 287 Middle East Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 288 Middle East Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 289 Middle East Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 290 Middle East Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 291 Middle East rica Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 292 Middle East Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 293 UAE Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 294 UAE Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 295 UAE Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 296 UAE Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 297 UAE Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 298 UAE Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 299 UAE Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 300 UAE Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 301 UAE Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 302 UAE Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 303 Saudi Arabia Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 304 Saudi Arabia Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 305 Saudi Arabia Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 306 Saudi Arabia Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 307 Saudi Arabia Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 308 Saudi Arabia Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 309 Saudi Arabia Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 310 Saudi Arabia Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 311 Saudi Arabia Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 312 Saudi Arabia Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 313 Rest of Middle East Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 314 Rest of Middle East Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 315 Rest of Middle East Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 316 Rest of Middle East Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 317 Rest of Middle East Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 318 Rest of Middle East Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 319 Rest of Middle East Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 320 Rest of Middle East Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 321 Rest of Middle East Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 322 Rest of Middle East Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 323 Africa Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 324 Africa Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 325 Africa Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 326 Africa Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 327 Africa Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 328 Africa Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 329 Africa Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 330 Africa Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 331 Africa Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 332 Africa Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 333 South Africa Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 334 South Africa Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 335 South Africa Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 336 South Africa Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 337 South Africa Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 338 South Africa Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 339 South Africa Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 340 South Africa Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 341 South Africa Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 342 South Africa Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 343 Egypt Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 344 Egypt Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 345 Egypt Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 346 Egypt Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 347 Egypt Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 348 Egypt Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 349 Egypt Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 350 Egypt Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 351 Egypt Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 352 Egypt Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)
TABLE 353 Rest of Africa Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2019-2023 (US$ Mn)
TABLE 354 Rest of Africa Acquired Hemophilia Treatment Market, by Factor Replacement Therapy, 2024-2032 (US$ Mn)
TABLE 355 Rest of Africa Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2019-2023 (US$ Mn)
TABLE 356 Rest of Africa Acquired Hemophilia Treatment Market, by Immunosuppressive Therapy, 2024-2032 (US$ Mn)
TABLE 357 Rest of Africa Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2019-2023 (US$ Mn)
TABLE 358 Rest of Africa Acquired Hemophilia Treatment Market, by Plasmapheresis and Immunoadsorption, 2024-2032 (US$ Mn)
TABLE 359 Rest of Africa Acquired Hemophilia Treatment Market, by Supportive Care, 2019-2023 (US$ Mn)
TABLE 360 Rest of Africa Acquired Hemophilia Treatment Market, by Supportive Care, 2024-2032 (US$ Mn)
TABLE 361 Rest of Africa Acquired Hemophilia Treatment Market, by Treatment Setting, 2019-2023 (US$ Mn)
TABLE 362 Rest of Africa Acquired Hemophilia Treatment Market, by Treatment Setting, 2024-2032 (US$ Mn)