Market Overview
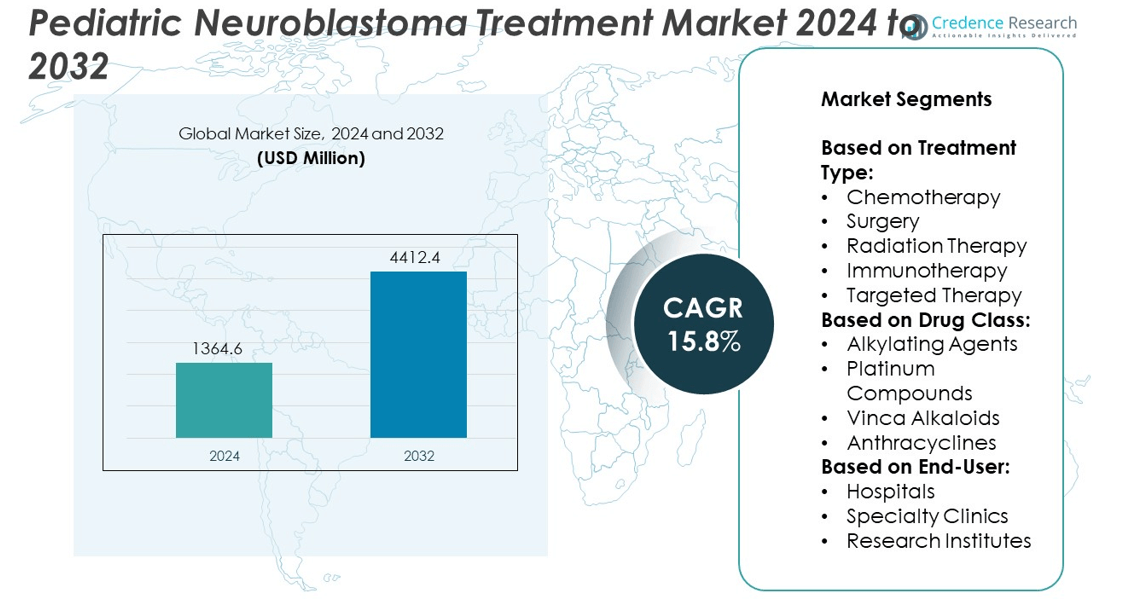
The Pediatric Neuroblastoma Treatment Market size was valued at USD 1364.6 million in 2024 and is anticipated to reach USD 4412.4 million by 2032, at a CAGR of 15.8% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Pediatric Neuroblastoma Treatment Market Size 2024 |
USD 1364.6 million |
| Pediatric Neuroblastoma Treatment Market, CAGR |
15.8% |
| Pediatric Neuroblastoma Treatment Market Size 2032 |
USD 4412.4 million |
The Pediatric Neuroblastoma Treatment market is driven by advancements in molecular diagnostics, rising adoption of immunotherapy, and increasing public and private funding for pediatric oncology research. Early genetic profiling enables risk-adapted treatment, while targeted therapies and monoclonal antibodies improve survival in high-risk patients. Trends include growing integration of personalized medicine, expansion of radiopharmaceutical applications, and increased participation in multicenter clinical trials. It reflects a shift toward precision-based approaches and collaborative innovation.
North America leads the Pediatric Neuroblastoma Treatment market due to advanced healthcare infrastructure, strong research institutions, and high adoption of targeted therapies. Europe follows with robust regulatory support and coordinated clinical trial networks. Asia-Pacific shows rising growth through improved diagnostics and government investment in pediatric oncology. Latin America and the Middle East & Africa are gradually expanding with public healthcare initiatives and global partnerships. Key players shaping the market include Y-mAbs Therapeutics, Inc, Bayer AG, and Cellectar Biosciences, Inc. These companies focus on developing innovative biologics and radiopharmaceuticals to enhance treatment outcomes in high-risk pediatric neuroblastoma cases.
Market Insights
- The Pediatric Neuroblastoma Treatment market was valued at USD 1364.6 million in 2024 and is expected to reach USD 4412.4 million by 2032, growing at a CAGR of 15.8% during the forecast period.
- Increasing use of genetic profiling and molecular diagnostics has enabled early detection and risk-based treatment, improving patient outcomes in pediatric neuroblastoma.
- Adoption of immunotherapy and targeted agents, including monoclonal antibodies and ALK inhibitors, is reshaping standard care protocols for high-risk and relapsed cases.
- Key players such as Y-mAbs Therapeutics, Bayer AG, and Cellectar Biosciences focus on advancing radiopharmaceuticals, antibody-based therapies, and precision oncology platforms.
- High treatment-related toxicity and long-term side effects pose clinical challenges, often requiring intensive supportive care and long-term monitoring in survivors.
- North America leads in innovation and access, followed by Europe with coordinated research networks, while Asia-Pacific shows promising growth due to rising healthcare investments.
- Industry trends reflect increased clinical trial collaboration, growing interest in personalized medicine, and supportive regulatory frameworks promoting pediatric drug development.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers
Advancements in Molecular Diagnostics and Genetic Profiling Expand Treatment Scope
The Pediatric Neuroblastoma Treatment market benefits significantly from advances in molecular diagnostics and genetic profiling. These technologies enable early detection of high-risk tumors through markers such as MYCN amplification and ALK mutations. Oncologists can tailor therapies based on individual tumor biology, improving treatment precision and outcomes. Diagnostic innovations support risk stratification, allowing clinicians to escalate or de-escalate interventions. It enhances survival rates and reduces long-term toxicity for pediatric patients. The adoption of next-generation sequencing (NGS) platforms further drives demand for targeted therapies.
- For instance, Y-mAbs Therapeutics reported an objective response rate of 34% in bone and bone marrow lesions from its Phase 2 naxitamab trial (NCT03326166), supporting its integration into relapsed neuroblastoma protocols.
Targeted Therapy and Immunotherapy Adoption Shifts Clinical Practice Patterns
Rising preference for targeted therapy and immunotherapy is reshaping treatment protocols in the Pediatric Neuroblastoma Treatment market. Monoclonal antibodies such as dinutuximab have shown improved survival in children with high-risk neuroblastoma. Immune checkpoint inhibitors and CAR-T cell therapies are entering clinical pipelines, offering new hope for refractory cases. It signals a shift away from conventional chemotherapy toward precision-based approaches. Pharmaceutical companies continue to invest in research partnerships to accelerate biologic approvals. The trend reflects clinician and caregiver demand for less invasive and more effective therapies.
- For instance, Qarziba® (dinutuximab beta), developed in collaboration with Apeiron Biologics AG and administered as maintenance immunotherapy with isotretinoin in the first-line high-risk setting, achieved a five-year event-free survival rate of 57% and a five-year overall survival rate of 64% in a cohort of 378 patients, according to the SIOPEN study.
Government Funding and Research Grants Drive Innovation in Pediatric Oncology
Increased public and private funding for pediatric cancer research supports clinical development in the Pediatric Neuroblastoma Treatment market. National initiatives, such as the U.S. RACE for Children Act, mandate pediatric evaluation of adult oncology drugs. Research institutions benefit from grants that enable early-phase trials of novel agents. It facilitates rapid translation of laboratory insights into clinical therapies. Collaborative networks among academic centers and pediatric hospitals further streamline innovation. Financial support improves drug accessibility and accelerates regulatory pathways.
Growing Awareness and Advocacy Improve Diagnosis and Early Intervention
Awareness campaigns and patient advocacy contribute to improved diagnosis rates across the Pediatric Neuroblastoma Treatment market. Parents and healthcare providers show increased vigilance in recognizing early symptoms such as abdominal swelling and fatigue. Advocacy groups work with healthcare systems to push for standardized screening protocols. It shortens diagnostic delays and increases access to specialized care centers. Timely intervention improves survival prospects and enhances quality of life. Awareness efforts continue to influence healthcare policies and patient navigation programs.
Market Trends
Expansion of Immunotherapeutic Modalities Reflects a Shift in Standard Care Approaches
The Pediatric Neuroblastoma Treatment market is witnessing a marked shift toward immunotherapy, with novel antibody-based therapies and cell therapies gaining traction. Agents like anti-GD2 monoclonal antibodies are being integrated into frontline treatment regimens. Clinical trials continue to assess CAR-T cell therapies and bispecific T-cell engagers in relapsed or refractory neuroblastoma. It reinforces a transition from cytotoxic agents to immune-based protocols. Regulatory bodies support fast-track approvals for promising pediatric immunotherapies. Pharmaceutical collaborations with research hospitals help bring these innovations to market faster.
- For instance, United Therapeutics Corporation’s Unituxin® (dinutuximab), when combined with GM-CSF, IL-2, and isotretinoin in the Phase III ANBL0032 trial, improved two-year event-free survival from 46 ± 5% to 66 ± 5% in children with high-risk neuroblastoma.
Personalized Medicine Gains Importance Through Biomarker-Driven Drug Development
The integration of genomic insights into treatment decisions is transforming the Pediatric Neuroblastoma Treatment market. Drug development strategies now emphasize biomarkers such as ALK mutations and Trk fusions. It supports precision oncology, where therapies align closely with tumor-specific genetic profiles. Companion diagnostics are being co-developed alongside targeted therapies to ensure clinical efficacy. These tools enable clinicians to predict patient response and reduce adverse events. Precision-based approaches continue to gain regulatory and clinical acceptance.
- For instance, Bayer AG’s larotrectinib (Vitrakvi®) achieved an overall response rate of 60% (with 24% complete responses and 36% partial responses), based on pooled data from three trials—LOXO-TRK-14001, SCOUT, and NAVIGATE—and demonstrated a median duration of response of 43.3 months, as reported in their FDA full approval announcement.
Focus on Combination Therapy Protocols Enhances Survival in High-Risk Patients
Oncology centers are adopting combination therapies involving chemotherapy, surgery, radiotherapy, and immunotherapy to improve outcomes in high-risk neuroblastoma. This trend reflects an evidence-based approach to managing tumor recurrence and progression. It improves event-free survival and overall remission rates in complex cases. Clinical guidelines increasingly recommend multimodal interventions tailored to patient risk categories. Research supports the use of maintenance therapy with isotretinoin and immunotherapeutics post-consolidation. Hospitals and cancer centers implement standardized protocols based on evolving trial data.
Global Collaborations and Multicenter Trials Strengthen Innovation Pipelines
The Pediatric Neuroblastoma Treatment market benefits from cross-border collaborations among academic institutions, pharmaceutical firms, and regulatory agencies. Multicenter trials enable faster patient enrollment and broader data validation. It accelerates the development of novel compounds and treatment algorithms. Shared databases and harmonized protocols improve trial reproducibility and global access. Organizations like the International Society of Paediatric Oncology (SIOPEN) drive these collaborative efforts. Such alliances support uniform standards of care and bring advanced treatments to diverse geographies.
Market Challenges Analysis
High Treatment Toxicity and Long-Term Side Effects Impact Quality of Life in Survivors
The Pediatric Neuroblastoma Treatment market faces a persistent challenge in managing the toxicity of intensive treatment regimens. High-dose chemotherapy, radiation, and immunotherapy often lead to long-term side effects, including hearing loss, growth delays, and organ dysfunction. It places a heavy burden on survivors, requiring lifelong monitoring and supportive care. The need to balance treatment efficacy with reduced toxicity remains unmet, particularly for high-risk patients. Pediatric protocols lack uniform standards for minimizing late effects without compromising remission rates. Clinicians and researchers continue to seek therapeutic approaches that preserve survival outcomes while improving post-treatment quality of life.
Limited Commercial Incentives and Research Barriers Slow Pediatric Drug Development
Pharmaceutical investment in rare pediatric cancers remains constrained due to low commercial returns and complex trial requirements. The Pediatric Neuroblastoma Treatment market struggles to attract sustained funding for novel drug development. It faces logistical hurdles in conducting large-scale trials, given the limited patient pool and ethical considerations. Regulatory frameworks demand high safety standards, extending development timelines and increasing costs. Smaller biotech firms often rely on academic partnerships to advance pipeline assets, but resource gaps delay progress. The slow pace of innovation restricts access to breakthrough therapies for children with relapsed or refractory disease.
Market Opportunities
Advances in Radiopharmaceuticals and Theranostics Create New Treatment Avenues
The Pediatric Neuroblastoma Treatment market holds strong potential through the integration of radiopharmaceuticals and theranostic platforms. Iodine-131 metaiodobenzylguanidine (I-131 MIBG) therapy has demonstrated success in treating relapsed cases with minimal systemic toxicity. It opens new opportunities for combining targeted radiation with systemic treatments. Emerging radiolabeled compounds with improved tumor selectivity offer further clinical promise. Diagnostic-imaging agents that double as therapeutic tools enable real-time monitoring of tumor response. These technologies improve disease management and support broader clinical adoption in high-risk patients.
Regulatory Incentives and Orphan Drug Designations Encourage Pipeline Expansion
Supportive regulatory pathways such as orphan drug status and priority review vouchers incentivize pharmaceutical firms to invest in rare pediatric oncology. The Pediatric Neuroblastoma Treatment market benefits from policies that reduce development barriers and accelerate time-to-approval. It allows smaller biotechs and academic institutions to advance therapies with fewer financial constraints. Government-backed programs in the U.S. and Europe promote early-stage innovation and cross-sector collaboration. Expanded access initiatives and compassionate use policies help bring experimental drugs to critically ill children. These mechanisms create favorable conditions for portfolio growth and address unmet clinical needs.
Market Segmentation Analysis:
By Treatment Type:
The Pediatric Neuroblastoma Treatment market categorizes by treatment type into chemotherapy, surgery, radiation therapy, immunotherapy, and targeted therapy. Chemotherapy remains the backbone of care, particularly in high-risk and metastatic cases. Multidrug regimens are standard for induction, consolidation, and maintenance phases. Surgery plays a critical role in tumor resection, with outcomes improving when combined with preoperative chemotherapy. Radiation therapy supports local control in residual or inoperable disease, especially in advanced stages. Immunotherapy, led by anti-GD2 monoclonal antibodies, gains traction due to its ability to boost survival without added toxicity. Targeted therapies show promise in relapsed cases, focusing on genetic mutations such as ALK and Trk fusions.
- For instance, proton beam therapy at Massachusetts General Hospital achieved local control in 28 out of 31 pediatric neuroblastoma patients over a median follow-up of 38 months, with reduced risk of secondary malignancies compared to conventional photon therapy.
By Drug Class:
Alkylating agents dominate the drug class segment, forming the core of frontline protocols. These include cyclophosphamide and ifosfamide, which disrupt DNA replication in rapidly dividing cancer cells. Platinum compounds such as cisplatin and carboplatin offer high efficacy but require renal and auditory monitoring. The Pediatric Neuroblastoma Treatment market also relies on vinca alkaloids like vincristine, valued for their mitotic inhibition with manageable side effects. Anthracyclines such as doxorubicin remain essential in multidrug regimens but carry cardiotoxicity risks, prompting research into liposomal formulations. Each drug class contributes differently to risk-adapted strategies across disease stages.
- For instance, Mylan’s vincristine, included in induction protocols alongside cyclophosphamide and topotecan, demonstrated measurable disease reduction in 29 out of 35 patients in Children’s Oncology Group pilot trials.
By End-User:
Hospitals lead the end-user segment, handling the majority of neuroblastoma diagnoses, surgical interventions, and chemotherapy administration. These centers offer integrated care teams, access to intensive care units, and clinical trial enrollment. Specialty clinics support outpatient follow-up, immunotherapy infusions, and survivorship care, providing continuity beyond the hospital setting. Research institutes play a key role in drug development, translational studies, and early-phase trials. It accelerates the pipeline for novel agents and supports evidence-based evolution in treatment protocols. Academic centers and children’s hospitals continue to anchor innovation and multidisciplinary care delivery in this market.
Segments:
Based on Treatment Type:
- Chemotherapy
- Surgery
- Radiation Therapy
- Immunotherapy
- Targeted Therapy
Based on Drug Class:
- Alkylating Agents
- Platinum Compounds
- Vinca Alkaloids
- Anthracyclines
Based on End-User:
- Hospitals
- Specialty Clinics
- Research Institutes
Based on the Geography:
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis
North America
North America holds the largest share of the Pediatric Neuroblastoma Treatment market, accounting for 43.7% in 2024. The region benefits from advanced pediatric oncology infrastructure, strong regulatory support, and high awareness among caregivers and clinicians. The United States leads in early diagnosis and adoption of high-intensity therapies such as immunotherapy and targeted agents. It hosts a large number of pediatric cancer research centers, including St. Jude Children’s Research Hospital and Dana-Farber Cancer Institute, which actively participate in clinical trials and translational studies. Government initiatives like the RACE for Children Act accelerate pediatric drug approvals by mandating pediatric investigation plans for adult oncology drugs. Canada also contributes to regional dominance through access to public healthcare and centralized cancer care programs. Insurance coverage and clinical collaboration between children’s hospitals support the region’s continued leadership in both access and innovation.
Europe
Europe captures 28.5% of the global Pediatric Neuroblastoma Treatment market, supported by a well-developed healthcare system and robust cross-border research initiatives. Countries such as Germany, France, and the United Kingdom are central to the region’s clinical advancements in pediatric oncology. The European Medicines Agency (EMA) promotes early authorization for orphan drugs targeting childhood cancers, which aids timely access to advanced therapies. It also benefits from networks like SIOPEN (International Society of Paediatric Oncology European Neuroblastoma), which coordinate multicenter trials and treatment standardization. Public healthcare coverage ensures equitable access to high-cost treatments like monoclonal antibodies and targeted drugs. Research hospitals and university-affiliated clinics play an instrumental role in delivering specialized care and expanding trial enrollment across Europe.
Asia-Pacific
Asia-Pacific represents 16.2% of the Pediatric Neuroblastoma Treatment market, with rising contributions from countries like Japan, China, and South Korea. The region experiences an upward trend in diagnosis and treatment due to improving healthcare infrastructure and expanding health insurance coverage. Japan’s early adoption of precision medicine and consistent investment in pediatric oncology helps drive innovation. China has increased funding for rare pediatric diseases, including neuroblastoma, through government-backed programs such as the Healthy China 2030 initiative. It sees rapid growth in hospital expansions and cancer screening programs. South Korea contributes through advanced biologics manufacturing and government incentives for pharmaceutical R&D. It reflects growing regional focus on early detection, genetic screening, and inclusion in global clinical studies.
Latin America
Latin America holds a smaller share at 7.4% but shows emerging potential in pediatric oncology development. Brazil and Mexico lead the region in terms of clinical infrastructure, patient volume, and pediatric oncology training programs. The Pediatric Neuroblastoma Treatment market in this region faces disparities in healthcare access and delayed diagnosis, particularly in rural areas. It benefits from increasing government focus on rare diseases and expansion of public healthcare programs. International partnerships with North American and European hospitals aid technology transfer and capacity building. Awareness campaigns and nonprofit initiatives contribute to early detection and treatment availability, gradually strengthening the market position across Latin America.
Middle East & Africa
The Middle East & Africa account for 4.2% of the global Pediatric Neuroblastoma Treatment market, constrained by limited infrastructure and low diagnostic penetration. Countries like the UAE, Saudi Arabia, and South Africa show gradual improvements through investment in pediatric specialty care and oncology research. It relies on support from global health organizations and private hospitals to provide access to advanced therapies. Diagnostic delays and lack of trained specialists remain challenges in many parts of the region. Efforts are underway to develop pediatric cancer registries and strengthen clinical trial participation. Growth remains moderate but sustained due to national cancer control strategies and external collaborations aiming to bridge the access gap.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
Competitive Analysis
The Pediatric Neuroblastoma Treatment market features strong competition among leading players such as Y-mAbs Therapeutics, Inc, Bayer AG, Pfizer Inc, Apeiron Biologics AG, Baxter, United Therapeutics Corporation, Cellectar Biosciences, Inc, Clarity Pharmaceuticals, Provectus Biopharmaceuticals, Inc, and Scorpion Therapeutics. These companies focus on targeted therapies, immunotherapy innovations, and radiopharmaceutical development to address high-risk and relapsed neuroblastoma cases. Pipeline expansion remains a central strategy, with firms advancing monoclonal antibodies, ALK inhibitors, and tumor-selective radioligands. Partnerships with pediatric research institutions and oncology centers enable accelerated clinical trial execution and regulatory engagement. Competitive differentiation is driven by product efficacy, safety profiles, and the ability to meet unmet clinical needs in the pediatric population. Several players secure orphan drug designations and breakthrough therapy statuses to expedite market entry. Investments in companion diagnostics and precision platforms also contribute to competitive positioning. Companies compete to improve survival rates, reduce treatment toxicity, and expand global access to advanced therapies. Strategic licensing, mergers, and acquisitions further strengthen market presence, particularly in North America and Europe. Market participants that successfully align clinical innovation with regulatory compliance and collaborative R&D models are expected to maintain or expand their market share in this rapidly evolving therapeutic area.
Recent Developments
- In 2025, Cellectar Biosciences, Inc.Reported initial positive outcomes from the CLOVER‑2 Phase 1 clinical trial of iopofosine I‑131 in pediatric high‑grade glioma, including extended progression-free survival and survival.
- In 2025, Y‑mAbs Therapeutics, Inc. Published interim Phase 2 results for naxitamab in treating relapsed/refractory high‑risk neuroblastoma.
- In August 2023, RENAISSANCE LAKEWOOD, LLC, a UK-based company that specializes in developing therapeutics for rare pediatric diseases, signed an exclusive license agreement.
Market Concentration & Characteristics
The Pediatric Neuroblastoma Treatment market demonstrates a moderately concentrated structure, with a limited number of specialized players driving innovation and commercialization. It is characterized by high entry barriers due to complex regulatory requirements, high development costs, and the need for pediatric-specific clinical data. The market focuses heavily on targeted therapies, immunotherapies, and radiopharmaceuticals, supported by academic collaborations and orphan drug incentives. Companies concentrate their efforts on rare, high-risk patient subsets where standard treatments show limited efficacy. Strong reliance on clinical trial outcomes and regulatory approvals shapes competitive dynamics, with product differentiation based on safety, efficacy, and access to advanced diagnostics. The market also exhibits high research intensity and a long development timeline, often requiring cross-sector partnerships for drug development and delivery. Large pharmaceutical firms and emerging biotechs both compete for leadership, with each leveraging distinct capabilities in innovation, manufacturing, and regulatory navigation. Hospitals and academic research institutes play a key role in treatment deployment and innovation pipelines, creating a multi-stakeholder environment. It remains highly responsive to scientific advancements, health policy changes, and shifts in research funding, reinforcing the need for adaptive, data-driven strategies to succeed in this specialized oncology segment.
Report Coverage
The research report offers an in-depth analysis based on Treatment Type, Drug Class, End-User and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- Immunotherapy will continue to expand, with new monoclonal antibodies and cell-based therapies expected to enter standard treatment protocols.
- Radiopharmaceuticals will gain clinical relevance, particularly for relapsed and high-risk neuroblastoma cases.
- Precision medicine will guide treatment selection through increased use of genetic profiling and companion diagnostics.
- More pharmaceutical firms will pursue pediatric-specific drug pipelines supported by regulatory incentives and orphan designations.
- Combination therapy regimens will become more refined to balance efficacy with long-term safety in young patients.
- Multicenter clinical trials will increase, enhancing data quality and accelerating regulatory approvals.
- Asia-Pacific will see significant market expansion driven by healthcare infrastructure development and clinical research participation.
- Hospitals and academic centers will strengthen partnerships with biotech firms to co-develop novel therapies.
- Digital health tools and AI-driven platforms will support early diagnosis and risk stratification in pediatric oncology.
- Survivor care models will evolve, focusing on minimizing late treatment effects and improving quality of life outcomes.




