Market Overview:
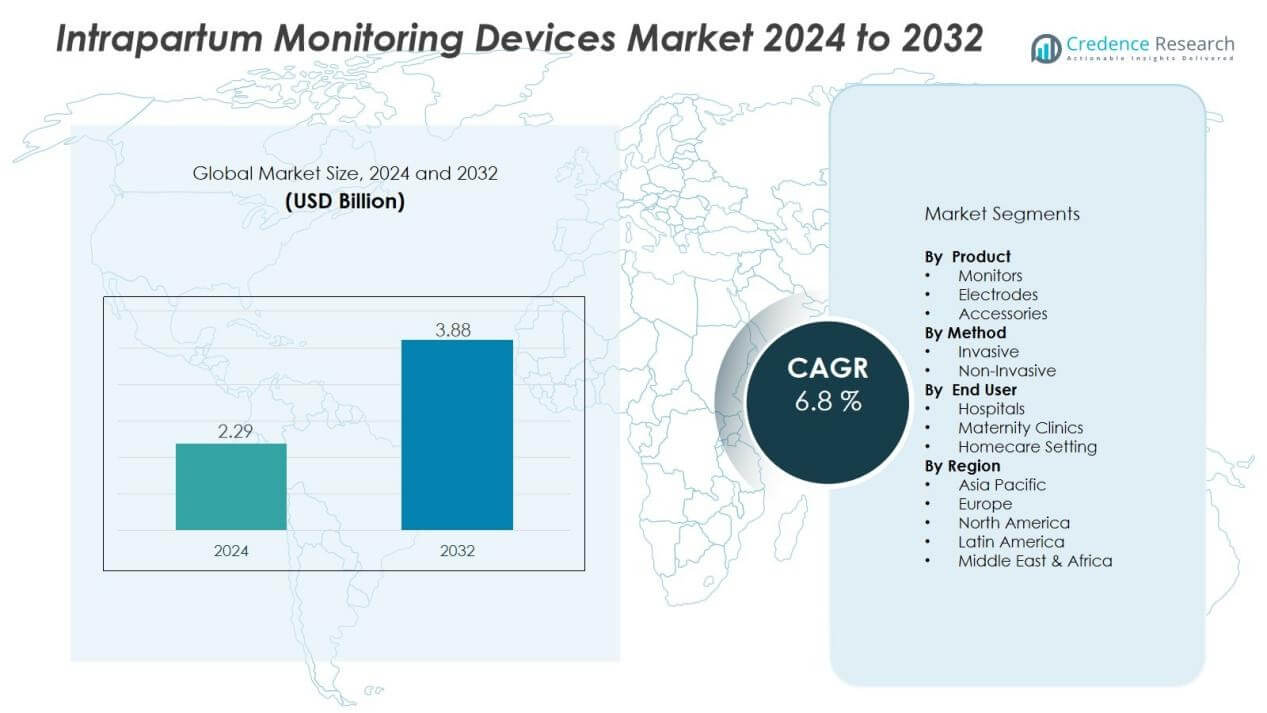
The intrapartum monitoring devices market size was valued at USD 2.29 billion in 2024 and is anticipated to reach USD 3.88 billion by 2032, at a CAGR of 6.8% during the forecast period (2024-2032).
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Intrapartum Monitoring Devices Market Size 2024 |
USD 2.29 Billion |
| Intrapartum Monitoring Devices Market, CAGR |
6.8% |
| Intrapartum Monitoring Devices Market Size 2032 |
USD 3.88 Billion |
Key drivers include the rising prevalence of pregnancy complications, preterm births, and maternal health risks. Increasing awareness of early detection of fetal distress and improvements in maternal care protocols are accelerating adoption. Government initiatives to reduce maternal and neonatal mortality, alongside higher healthcare spending, further strengthen market growth. Integration of artificial intelligence and real-time data analytics into monitoring systems is also reshaping clinical decision-making.
Regionally, North America leads the intrapartum monitoring devices market due to advanced healthcare infrastructure, favorable reimbursement policies, and strong presence of key medical device companies. Europe follows closely, supported by robust maternal healthcare programs and clinical adoption of digital monitoring technologies. Asia-Pacific is anticipated to witness the fastest growth, driven by a high birth rate, increasing healthcare investments, and rising maternal health awareness in countries such as India and China. Latin America and the Middle East & Africa represent emerging opportunities with expanding healthcare access.
Market Insights:
- The intrapartum monitoring devices market was valued at USD 2.29 billion in 2024 and is expected to reach USD 3.88 billion by 2032, growing at a CAGR of 6.8%.
- Rising pregnancy complications, preterm births, and maternal health risks are driving adoption of advanced monitoring systems.
- Government programs to reduce maternal and neonatal mortality, combined with higher healthcare spending, are reinforcing demand.
- Technological advancements in wireless, portable, and AI-enabled devices are improving clinical decision-making and patient safety.
- High costs and limited accessibility in low-resource settings continue to challenge broader adoption, especially in rural areas.
- North America holds 38% market share in 2024, supported by strong infrastructure, reimbursement policies, and technology adoption.
- Europe accounts for 29% market share, while Asia-Pacific holds 22% and records the fastest growth driven by high birth rates and expanding healthcare access.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers:
Rising Prevalence of Pregnancy Complications and Preterm Births:
The increasing number of pregnancy complications and preterm births strongly supports the adoption of intrapartum monitoring devices. Early detection of fetal distress and maternal health risks has become a critical part of modern obstetric care. Healthcare providers rely on continuous monitoring to reduce adverse outcomes and improve delivery safety. The intrapartum monitoring devices market benefits from the urgent need for real-time insights during labor.
- For instance, the implementation of the Moyo continuous fetal Doppler device in a Tanzanian tertiary hospital increased the detection of abnormal fetal heart rates by 6.9 times, enabling more timely obstetric interventions to improve neonatal outcomes.
Growing Emphasis on Maternal and Neonatal Safety Standards:
Government initiatives and global health programs are strengthening maternal and neonatal safety protocols. Hospitals and maternity clinics integrate advanced monitoring systems to align with these standards. Improved clinical guidelines encourage routine use of monitoring devices to minimize risks. It reinforces demand by ensuring healthcare systems prioritize patient outcomes and preventive care.
- For instance, the Nemo Fetal Monitoring System from Nemo Healthcare BV achieved a fetal heart rate signal acquisition success rate of 99.4 percent with a root mean square error of 5.1 beats per minute during intrapartum monitoring.
Technological Advancements in Wireless and Portable Monitoring Solutions:
Innovation in device design has transformed how monitoring is conducted during childbirth. Portable and wireless devices increase comfort for mothers while enabling continuous observation. Integration of data analytics and AI-driven alerts improves the accuracy of clinical decision-making. The intrapartum monitoring devices market benefits significantly from these advancements, enhancing accessibility across different healthcare settings.
Expanding Healthcare Investments and Infrastructure in Emerging Regions:
Developing countries are experiencing stronger investments in maternal healthcare infrastructure. Rising awareness of maternal health challenges encourages governments and private players to expand access to advanced monitoring technologies. Training programs for healthcare professionals further promote the use of these devices in clinical practice. It creates a favorable environment for market growth, especially in Asia-Pacific, Latin America, and Africa.
Market Trends:
Integration of Artificial Intelligence and Data-Driven Clinical Insights:
The adoption of artificial intelligence in intrapartum monitoring devices is reshaping clinical practice. AI-enabled systems improve interpretation of fetal heart rate patterns and uterine activity, reducing human error. Advanced analytics provide predictive insights that help clinicians make timely interventions. Hospitals are increasingly adopting cloud-based platforms that allow real-time data sharing between care teams. This trend enhances patient safety while reducing complications during delivery. The intrapartum monitoring devices market benefits from this transformation, with AI-driven tools gaining acceptance among obstetricians. It reinforces trust in technology-enabled maternal and neonatal care.
- For Instance, PeriGen’s FDA-cleared Patterns 3.0 fetal monitoring software extends its AI-driven fetal heart rate pattern recognition technology to 32 weeks of gestation, allowing for earlier monitoring of high-risk pregnancies.
Shift Toward Wireless, Portable, and Patient-Centric Monitoring Solutions:
The demand for wireless and portable monitoring devices is increasing rapidly in maternity care. These solutions allow mothers greater mobility during labor, improving comfort without compromising safety. Portable devices also support broader adoption in resource-limited settings where traditional systems are less accessible. The intrapartum monitoring devices market is witnessing strong momentum from innovations that prioritize user-friendly design. Healthcare providers are adopting wearable sensors and handheld monitors to expand access to quality care. It aligns with the global focus on patient-centric models, ensuring both clinical efficiency and enhanced patient experience. This trend is expected to drive long-term adoption across both developed and emerging regions.
Market Challenges Analysis:
High Costs and Limited Accessibility in Low-Resource Settings:
The cost of advanced intrapartum monitoring devices remains a significant barrier for widespread adoption. Many healthcare facilities in low- and middle-income countries struggle to afford modern monitoring systems. Limited healthcare budgets restrict investment in new technologies, delaying upgrades to outdated equipment. The intrapartum monitoring devices market faces uneven adoption, with rural and underfunded regions lagging behind. It creates disparities in maternal and neonatal outcomes, particularly where complications are more common. High procurement and maintenance expenses further discourage smaller clinics from adopting these devices.
Technical Complexity and Shortage of Skilled Healthcare Professionals:
The use of sophisticated monitoring devices requires skilled healthcare staff capable of accurate interpretation. A shortage of trained professionals limits effective utilization, particularly in developing healthcare systems. Technical complexity often leads to misinterpretation of fetal data, raising concerns about false alarms or unnecessary interventions. The intrapartum monitoring devices market must address training and usability challenges to ensure reliable outcomes. It highlights the need for simplified interfaces and continuous education programs for clinicians. Resistance to adopting unfamiliar technologies also slows down implementation in traditional healthcare settings. These barriers collectively impact the overall growth potential of the market.
Market Opportunities:
Rising Demand for Advanced Maternal and Neonatal Care Solutions:
The global focus on reducing maternal and neonatal mortality rates is creating strong opportunities for innovation. Governments and health organizations are investing in advanced technologies to improve labor monitoring standards. The intrapartum monitoring devices market can expand by offering solutions tailored for preventive care and early risk detection. It benefits from growing demand for wireless, portable, and AI-enabled devices that enhance accuracy. Hospitals and maternity clinics are adopting smart monitoring systems to strengthen patient outcomes. The shift toward evidence-based care supports faster adoption of advanced tools across developed regions.
Expanding Penetration in Emerging Economies with Healthcare Investments:
Emerging economies are increasing healthcare budgets and expanding access to maternity care infrastructure. Rising awareness of maternal health risks is encouraging demand for modern monitoring solutions. The intrapartum monitoring devices market has strong potential in Asia-Pacific, Latin America, and parts of Africa. It aligns with government initiatives that promote safe childbirth and improved clinical practices. Local partnerships and affordable device options can help companies capture underpenetrated markets. Strong investment in healthcare digitization further creates a pathway for innovative monitoring technologies to scale. These regions represent high-growth opportunities for global manufacturers seeking long-term expansion.
Market Segmentation Analysis:
By Product:
The intrapartum monitoring devices market by product is segmented into monitors, electrodes, and accessories. Monitors dominate the segment due to their essential role in continuous fetal and maternal observation. Electrodes are gaining importance for their accuracy in fetal heart rate detection. Accessories complement device usage and ensure reliable performance across clinical settings. It highlights the growing demand for advanced, integrated solutions to improve outcomes.
- For instance, the Philips Avalon CL wireless fetal monitor system achieves a reliable fetal and maternal vital signs signal over a line-of-sight operating range of up to 100 meters
By Method:
The market by method is divided into invasive and non-invasive techniques. Non-invasive monitoring holds the larger share due to patient comfort, reduced risk, and growing clinical preference. Invasive methods remain critical in high-risk pregnancies where precision is required. Hospitals are increasingly investing in advanced non-invasive systems with wireless connectivity. It reflects the trend toward safer and more efficient monitoring practices.
- For Instance,GE Healthcare’s Novii+ wireless maternal and fetal monitoring patch received FDA 510(k) clearance in February 2024 for use in pregnant patients from 34 weeks onward.
By End User:
The market by end user includes hospitals, maternity clinics, and homecare settings. Hospitals account for the largest share owing to advanced infrastructure and high patient volumes. Maternity clinics are expanding their adoption of portable and wireless solutions to improve maternal care. Homecare settings represent a growing opportunity driven by rising demand for personalized and convenient monitoring. It showcases how diverse healthcare environments are shaping adoption patterns across the intrapartum monitoring devices market.
Segmentations:
By Product
- Monitors
- Electrodes
- Accessories
By Method
By End User
- Hospitals
- Maternity Clinics
- Homecare Setting
By Region
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis:
North America:
North America holds 38% market share in the intrapartum monitoring devices market in 2024. It is supported by advanced healthcare infrastructure, strong presence of leading manufacturers, and favorable reimbursement policies. Hospitals and maternity centers in the United States and Canada prioritize advanced monitoring devices to ensure maternal and neonatal safety. Growing adoption of AI-enabled and wireless monitoring systems further drives regional expansion. Government initiatives focused on reducing maternal mortality rates reinforce market growth. The region also benefits from a high level of awareness and early adoption of innovative healthcare technologies.
Europe:
Europe accounts for 29% market share in the intrapartum monitoring devices market in 2024. The region demonstrates strong demand driven by well-established maternal care programs and widespread clinical adoption. Countries such as Germany, France, and the United Kingdom actively integrate advanced monitoring solutions into healthcare systems. Supportive government funding enhances the adoption of digital monitoring devices. Hospitals are prioritizing standardized maternal and neonatal safety protocols to improve outcomes. It benefits from growing use of cloud-based platforms that allow real-time collaboration across healthcare teams. This ensures consistent demand for reliable monitoring solutions.
Asia-Pacific:
Asia-Pacific holds 22% market share in the intrapartum monitoring devices market in 2024. It is expected to record the fastest growth due to rising healthcare investments, large patient base, and high birth rates. Countries such as China and India are expanding healthcare access with stronger maternal care initiatives. Growing awareness of neonatal health is accelerating adoption of modern monitoring technologies. Local manufacturing and affordable device options are further supporting penetration in developing markets. The region benefits from government-led programs aimed at improving maternal outcomes. It represents a key growth engine for global manufacturers targeting long-term expansion.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
Competitive Analysis:
The intrapartum monitoring devices market is characterized by strong competition among global and regional players. Key companies include Cardinal Health, Koninklijke Philips N.V., CooperSurgical, Inc., MindChild Medical, GE Healthcare, Olympus Corporation, and Medtronic plc. These firms focus on innovation, product development, and strategic collaborations to expand their presence in hospitals, maternity clinics, and homecare settings. It demonstrates high emphasis on integrating advanced technologies such as wireless monitoring, AI-enabled analysis, and cloud-based platforms to enhance clinical outcomes. Companies invest heavily in R&D to improve accuracy, patient comfort, and reliability of devices. Strategic acquisitions and partnerships are shaping competitive strategies, allowing players to strengthen distribution networks and extend reach in emerging economies. Continuous product launches with improved design and usability highlight the industry’s commitment to advancing maternal and neonatal care. This competitive landscape reflects sustained efforts to meet rising global demand for efficient intrapartum monitoring solutions.
Recent Developments:
- In November 2024, Cardinal Health launched the Kendall SCD SmartFlow™ Compression System in the U.S., designed to prevent venous thromboembolism with advanced compression therapy technology.
- In August 2025, Cardinal Health announced the acquisition of Solaris Health for approximately $1.9 billion in cash, expanding its specialty healthcare business.
Report Coverage:
The research report offers an in-depth analysis based on Product, Method, End User and Region. It details leading Market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current Market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven Market expansion in recent years. The report also explores Market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on Market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the Market.
Future Outlook:
- The intrapartum monitoring devices market will continue to evolve with stronger focus on maternal and neonatal safety.
- Integration of artificial intelligence will enhance accuracy in fetal heart rate and contraction analysis.
- Wireless and portable monitoring devices will gain wider adoption across hospitals and maternity clinics.
- Cloud-based platforms will support real-time data sharing and improve collaboration among healthcare professionals.
- Emerging economies will drive expansion through government investments in maternal health infrastructure.
- Patient-centric solutions will remain a priority, with devices designed for comfort and ease of use.
- Collaborations between technology companies and healthcare providers will accelerate innovation in monitoring systems.
- Growing awareness of early detection and prevention of complications will fuel consistent demand.
- Training programs and simplified device interfaces will reduce skill barriers for healthcare staff.
- Sustainability in manufacturing and cost-effective product designs will strengthen accessibility in low-resource settings.




