Market Overview
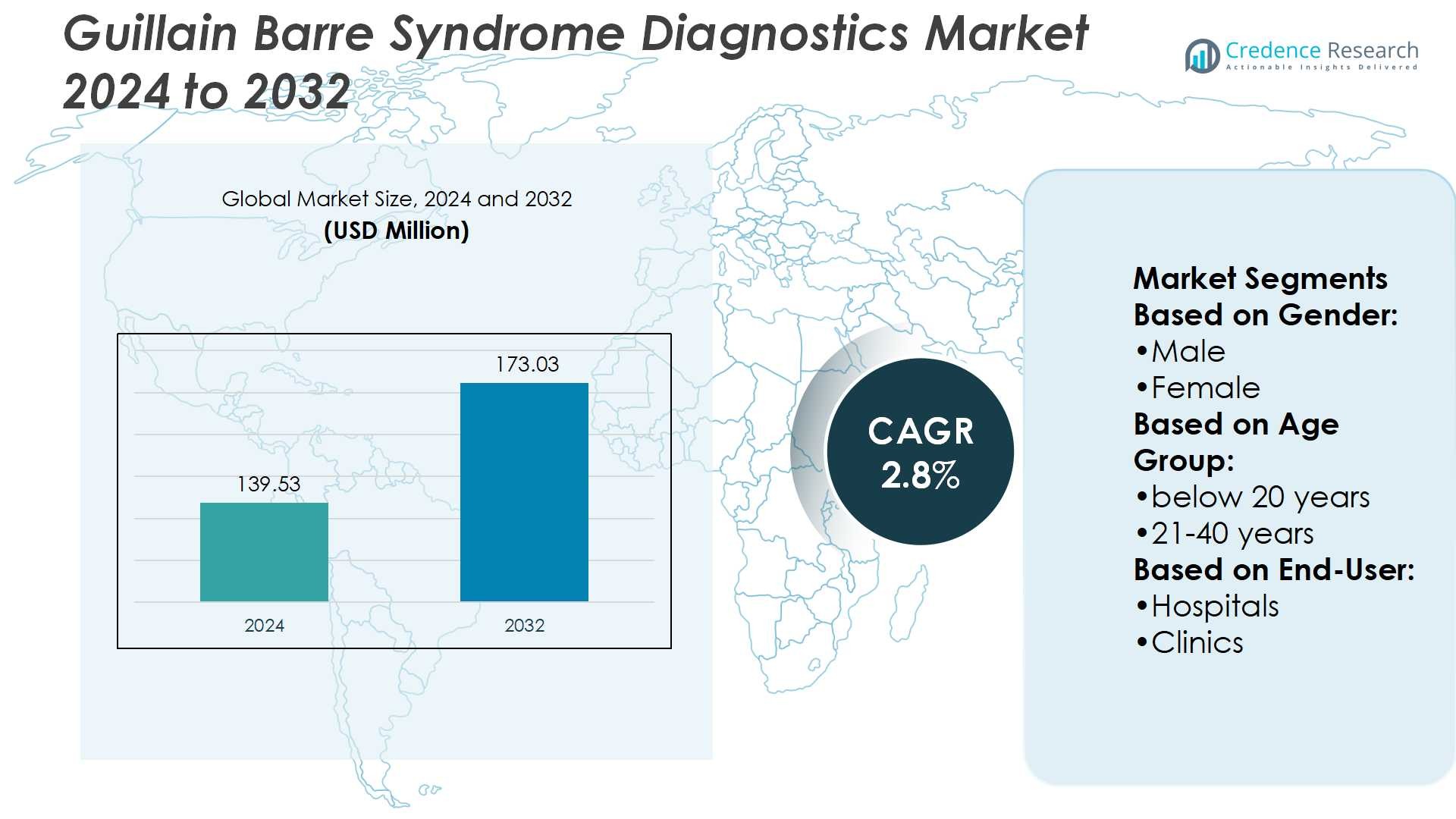
Guillain Barre Syndrome Diagnostics Market size was valued at USD 139.53 million in 2024 and is anticipated to reach USD 173.03 million by 2032, at a CAGR of 2.8% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Guillain Barre Syndrome Diagnostics Market Size 2024 |
USD 139.53 Million |
| Guillain Barre Syndrome Diagnostics Market, CAGR |
2.8% |
| Guillain Barre Syndrome Diagnostics Market Size 2032 |
USD 173.03 Million |
The Guillain Barre Syndrome Diagnostics Market is driven by rising global incidence of neurological disorders, growing demand for early detection, and expanding healthcare infrastructure supporting advanced testing. Increased use of nerve conduction studies, cerebrospinal fluid analysis, and biomarker-based assays enhances diagnostic reliability. Artificial intelligence and machine learning are being integrated to improve accuracy and speed, while point-of-care solutions expand access in emergency and resource-limited settings. Collaborative research between hospitals, academic institutes, and diagnostic firms strengthens innovation pipelines. Expanding public health initiatives and funding for neurological research further support adoption, shaping strong growth opportunities for advanced diagnostic solutions.
The Guillain Barre Syndrome Diagnostics Market shows strong regional presence, with Asia Pacific holding the largest share, followed by North America and Europe due to advanced healthcare systems and research investments, while Latin America and the Middle East & Africa grow steadily with improving infrastructure. Key players shaping the market include AbbVie Inc., Biogen Inc., Cadila Healthcare Limited, CSL Behring LLC, F. Hoffmann-La Roche Ltd., GSK plc, Grifols SA, LGM Pharmaceuticals Inc., Merck & Co. Inc., and Octapharma AG.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The Guillain Barre Syndrome Diagnostics Market was valued at USD 139.53 million in 2024 and is projected to reach USD 173.03 million by 2032, growing at a CAGR of 2.8%.
- Rising incidence of neurological disorders and the growing demand for early detection are key drivers.
- Artificial intelligence, machine learning, and biomarker-based assays are emerging as strong diagnostic trends.
- Competitive activity focuses on innovation, partnerships, and expanding access to advanced neurodiagnostic tools.
- High diagnostic costs and limited awareness in low-resource regions act as major restraints.
- Asia Pacific holds the largest regional share, followed by North America and Europe, while Latin America and the Middle East & Africa show steady growth.
- Key players include AbbVie Inc., Biogen Inc., Cadila Healthcare Limited, CSL Behring LLC, F. Hoffmann-La Roche Ltd., GSK plc, Grifols SA, LGM Pharmaceuticals Inc., Merck & Co. Inc., and Octapharma AG.
Market Drivers
Rising Global Incidence of Neurological Disorders Increases Demand for Timely Diagnostics
The Guillain Barre Syndrome Diagnostics Market benefits from a rising prevalence of neurological conditions, particularly autoimmune disorders affecting the peripheral nervous system. Guillain Barre Syndrome (GBS) is often linked to viral and bacterial infections, which continue to rise globally. Timely and accurate diagnosis remains vital for reducing long-term disability and improving recovery outcomes. Hospitals and clinics are expanding diagnostic capacity to meet this demand. Public health organizations highlight the urgency of early detection to prevent severe complications. This dynamic drives continuous investment in diagnostic tools and methods.
- For instance, in the PATH extension study by CSL Behring, 82 adult CIDP patients were enrolled: 62 patients started on the 0.4 g/kg weekly dose of Hizentra and 20 started on the 0.2 g/kg weekly dose.
Advancements in Neurodiagnostic Technologies Enhance Accuracy and Speed
Growing innovation in neurodiagnostic tools supports expansion of the Guillain Barre Syndrome Diagnostics Market. Advanced techniques such as nerve conduction studies, cerebrospinal fluid analysis, and MRI provide higher sensitivity and specificity. Companies are focusing on devices that deliver rapid results and improved accuracy. It enables clinicians to differentiate GBS from other neuropathies more effectively. Investment in AI-assisted imaging and automated electrophysiology adds precision to patient evaluation. Rising adoption of such technologies strengthens confidence among healthcare providers.
- For instance, A machine-learning screening funnel predicting Alzheimer’s disease progression (change in Clinical Dementia Rating-Sum of Boxes >1 over two years) achieved area under the curve (AUC) of 0.836.
Increasing Awareness and Early Detection Programs Drive Adoption
Rising awareness campaigns and patient education programs strengthen the Guillain Barre Syndrome Diagnostics Market. Healthcare providers emphasize the importance of early detection to improve patient survival rates. Governments and non-profit organizations support awareness drives that encourage people to seek medical care at the onset of symptoms. Hospitals are integrating structured diagnostic pathways that prioritize speed and accuracy. Training programs for neurologists and general physicians further build diagnostic capacity. It contributes to better patient outcomes and reduced healthcare burden.
Expanding Healthcare Infrastructure and Research Funding Fuel Market Growth
The Guillain Barre Syndrome Diagnostics Market gains momentum from growing investments in healthcare infrastructure and research funding. Emerging economies are prioritizing neurological disease diagnostics by upgrading laboratory facilities. Research organizations are receiving grants to study GBS biomarkers and develop advanced assays. It expands opportunities for new product launches and collaborations between hospitals, biotech firms, and research institutes. Improved accessibility to diagnostic centers ensures broader patient coverage. Governments’ focus on strengthening healthcare delivery underpins steady growth in this space.
Market Trends
Integration of Artificial Intelligence and Machine Learning in Diagnostic Tools
The Guillain Barre Syndrome Diagnostics Market is witnessing rapid integration of artificial intelligence and machine learning. These technologies assist in interpreting electrophysiological tests and imaging results with greater accuracy. It enables physicians to detect subtle abnormalities that may otherwise be overlooked. Companies are developing AI-powered algorithms that can analyze nerve conduction patterns in real time. Cloud-based platforms are also being used to store and interpret patient data more efficiently. This trend strengthens diagnostic precision while reducing turnaround times for clinicians.
- For instance, Grifols’ plasma repository exceeds 100 million samples, collected over nearly 15 years, which AI and proteomics teams use to analyze thousands of proteins in longitudinal studies for early biomarkers.
Growing Use of Biomarkers and Molecular Diagnostic Approaches
The Guillain Barre Syndrome Diagnostics Market is influenced by the growing adoption of biomarkers and molecular testing. Researchers are focusing on specific immune-related markers in cerebrospinal fluid to support early detection. It improves the ability to differentiate GBS from other neuropathies. Pharmaceutical and diagnostic companies are investing in biomarker-based assays to provide reliable results. Molecular tests enhance the accuracy of clinical evaluations when combined with traditional techniques. This shift reflects the broader industry move toward personalized medicine and precision diagnostics.
- For instance, Octapharma enrolled 71 pediatric patients (age 6-17) in a randomized, double-blind, placebo-controlled Phase 3 trial of PANZYGA® (10% IVIG) for Pediatric Acute-Onset Neuropsychiatric Syndrome.
Expansion of Point-of-Care Diagnostic Solutions for Faster Access
The Guillain Barre Syndrome Diagnostics Market benefits from the expansion of point-of-care diagnostic solutions. Portable electrophysiology systems and rapid cerebrospinal fluid assays are being introduced for emergency use. It allows healthcare providers to identify the disorder in critical settings with minimal delay. Hospitals and clinics are adopting compact devices that support diagnosis outside traditional labs. This trend improves access to diagnostics in resource-limited regions. The growing demand for rapid, reliable, and mobile testing systems continues to shape investment priorities.
Increasing Collaborations Between Academic Institutes and Industry Players
The Guillain Barre Syndrome Diagnostics Market is shaped by rising collaborations between universities, hospitals, and diagnostic companies. Partnerships aim to accelerate the development of novel diagnostic protocols and improve existing technologies. It encourages joint clinical trials, data sharing, and standardization of diagnostic criteria. Public-private partnerships support funding for research projects on immune-mediated neuropathies. Companies gain access to large patient databases that improve test validation. This trend strengthens innovation pipelines and supports widespread adoption of advanced diagnostic practices.
Market Challenges Analysis
High Complexity of Diagnosis and Limited Differentiation from Other Neuropathies
The Guillain Barre Syndrome Diagnostics Market faces challenges due to the complex nature of the disorder and overlapping symptoms with other neuropathies. It often requires multiple tests such as nerve conduction studies, cerebrospinal fluid analysis, and MRI to confirm diagnosis. Delayed recognition can lead to severe complications and longer recovery periods. Physicians in smaller healthcare facilities struggle with access to advanced diagnostic tools, creating gaps in timely treatment. Lack of standardized diagnostic guidelines across regions further complicates consistency. These challenges reduce diagnostic efficiency and impact patient outcomes in critical cases.
Limited Awareness, High Costs, and Unequal Access to Diagnostic Infrastructure
The Guillain Barre Syndrome Diagnostics Market is restrained by limited awareness among patients and healthcare workers, particularly in low-resource regions. It contributes to underreporting and delays in identifying early-stage cases. Diagnostic procedures remain costly, especially when advanced neuroimaging or biomarker tests are required. Many healthcare systems face financial limitations, preventing wider adoption of new technologies. Unequal access to specialized neurologists and diagnostic centers limits availability in rural and underserved areas. These barriers create disparities in care delivery and slow down broader market penetration.
Market Opportunities
Rising Demand for Advanced Diagnostic Technologies and Early Detection Solutions
The Guillain Barre Syndrome Diagnostics Market presents significant opportunities through the rising demand for advanced technologies that support early detection. It allows healthcare providers to identify the condition before severe nerve damage occurs, improving treatment outcomes. Innovations in AI-driven electrophysiology, biomarker-based assays, and molecular testing create avenues for product development. Hospitals and diagnostic centers are showing strong interest in portable and point-of-care systems that deliver rapid results. Emerging economies are expanding healthcare budgets, enabling broader adoption of modern diagnostics. Companies investing in these technologies can secure strong positions in specialized neurology markets.
Expanding Research Collaborations and Public Health Initiatives Supporting Growth
The Guillain Barre Syndrome Diagnostics Market is positioned for growth through expanding collaborations among academic institutes, biotech firms, and hospitals. It strengthens research pipelines by supporting biomarker discovery, clinical trials, and validation studies. Governments and global health agencies are focusing on public health programs that raise awareness of autoimmune neuropathies. Funding support for neurological research encourages development of novel diagnostic protocols. Pharmaceutical companies entering strategic alliances with diagnostic players further boost innovation and commercialization. These opportunities highlight the potential for long-term advancements in diagnostic efficiency and accessibility.
Market Segmentation Analysis:
By Gender
The Guillain Barre Syndrome Diagnostics Market reflects a higher prevalence among males compared to females, which shapes diagnostic demand across healthcare systems. It is widely reported that male patients account for a larger proportion of diagnosed cases, driving hospitals and clinics to maintain strong capacity for early detection in this group. Female patients also remain a significant segment, with diagnostic requirements linked to pregnancy-related immune variations and post-infectious conditions. Increased awareness and routine neurological evaluations are contributing to balanced growth across both genders. Diagnostic providers continue to adapt strategies to ensure that both male and female patients receive accurate and timely evaluations.
- For instance, A study of 60 Guillain-Barré syndrome (GBS) patients at Tikur Anbessa Specialized Hospital in Ethiopia found a male predominance, with 37 (61.7%) of the patients being male and 23 (38.3%) being female.
By Age Group
The Guillain Barre Syndrome Diagnostics Market shows notable variation across age groups, with adults between 41 and 59 years representing a key diagnostic segment. It is often the age group most affected due to higher exposure to triggering infections and co-morbidities. Patients aged 60 years and above also account for a rising share, driven by aging populations and vulnerability to immune-related disorders. Younger populations, including those below 20 years, represent a smaller but growing segment where early detection is critical to prevent disability. The 21–40 years segment is expanding as improved awareness encourages timely neurological evaluations. This age-based segmentation highlights the need for tailored diagnostic approaches across different life stages.
- For instance, In the CLARITY-AD study, Biogen and Eisai evaluated lecanemab treatment, which reduced plasma p-tau217 levels over 18 months compared to placebo, confirming the value of molecular diagnostics in age-related neurodegenerative conditions.
By End-User
Hospitals dominate the Guillain Barre Syndrome Diagnostics Market due to their advanced neurodiagnostic infrastructure, including nerve conduction studies, cerebrospinal fluid testing, and MRI facilities. It allows hospitals to serve as referral centers for complex cases requiring immediate attention. Clinics represent an important segment for early-stage evaluations and routine follow-ups, often supporting hospitals in broader care delivery. Rehabilitation centers are gaining importance as GBS patients frequently require long-term recovery monitoring, physical therapy, and ongoing neurological assessments. Growing investment in rehabilitation facilities strengthens their role in supporting diagnostic follow-up and patient management. This end-user structure ensures a comprehensive pathway for detection, treatment, and recovery across the healthcare ecosystem.
Segments:
Based on Gender:
Based on Age Group:
- below 20 years
- 21-40 years
Based on End-User:
Based on the Geography:
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis
North America
North America accounts for 28% of the Guillain Barre Syndrome Diagnostics Market. The region benefits from advanced healthcare infrastructure, high awareness of neurological disorders, and strong adoption of modern diagnostic tools. The United States leads, supported by widespread insurance coverage and robust R&D investments in AI and biomarker-based assays. Canada also contributes with national healthcare systems ensuring early diagnostic access. Strong collaboration between hospitals, universities, and biotech firms enhances diagnostic accuracy. Despite high costs, North America remains a leader in innovation and clinical advancements.
Europe
Europe holds 22% of the global market share, driven by structured healthcare systems and extensive neurological research initiatives. Germany, the UK, and France dominate the region with advanced diagnostic centers. Strong regulatory frameworks support innovation but sometimes slow down adoption due to complex reimbursement policies. Hospitals across Europe continue to expand access to nerve conduction studies and cerebrospinal fluid analysis. Rising awareness among physicians and patients helps maintain steady demand. Public funding for research on immune-mediated disorders adds further momentum to diagnostic advancements.
Asia Pacific
Asia Pacific leads with 42% of the Guillain Barre Syndrome Diagnostics Market, supported by rapid healthcare expansion and rising disease prevalence. China, India, and Japan remain major contributors, with strong investments in diagnostic laboratories and neurophysiology tools. Governments allocate significant funds to improve early detection and awareness. Growing adoption of point-of-care diagnostic solutions expands access, especially in urban centers. Rising medical tourism further boosts regional demand. Asia Pacific’s dominance reflects both large patient pools and expanding healthcare infrastructure.
Latin America
Latin America accounts for 5% of the market, led by Brazil and Mexico. Hospitals in urban areas are increasing adoption of advanced diagnostic methods, while rural regions still face access challenges. Awareness programs are gradually improving recognition of early symptoms. Regional growth is supported by modernization of healthcare infrastructure and greater investment in neurology. Partnerships with North American and European companies provide technology transfer and training. The market is small but expected to expand steadily.
Middle East & Africa
The Middle East & Africa represents 3% of the Guillain Barre Syndrome Diagnostics Market. The region faces limitations due to uneven healthcare infrastructure and shortage of specialized neurologists. Gulf countries such as Saudi Arabia and the UAE invest heavily in advanced diagnostic centers, improving regional performance. Africa remains behind but is seeing growth through public health initiatives and international aid programs. Hospitals in major cities are adopting better diagnostic equipment, slowly narrowing access gaps. Long-term opportunities exist as healthcare reforms accelerate.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- CSL Behring LLC
- AbbVie Inc.
- Grifols SA
- Cadila Healthcare Limited
- Octapharma AG
- Merck & Co. Inc.
- GSK plc
- Biogen Inc.
- LGM Pharmaceuticals Inc.
- Hoffmann-La Roche Ltd.
Competitive Analysis
The Guillain Barre Syndrome Diagnostics Market companies include AbbVie Inc., Biogen Inc., Cadila Healthcare Limited, CSL Behring LLC, F. Hoffmann-La Roche Ltd., GSK plc, Grifols SA, LGM Pharmaceuticals Inc., Merck & Co. Inc., and Octapharma AG. The Guillain Barre Syndrome Diagnostics Market is defined by intense competition, with companies focusing on innovation, accessibility, and clinical accuracy. Firms are prioritizing advancements in neurodiagnostic technologies, including nerve conduction studies, biomarker assays, and molecular testing, to strengthen diagnostic reliability. Strategic collaborations between pharmaceutical developers, diagnostic firms, and academic institutions are expanding research pipelines and improving standardization of testing protocols. Investment in emerging economies continues to rise, aiming to enhance diagnostic reach in underserved regions and reduce disparities in patient care. Market players also emphasize affordability and speed of diagnosis, aligning product strategies with the growing demand for early detection and precision medicine. This competitive landscape highlights continuous efforts to integrate advanced technologies, expand global presence, and deliver comprehensive solutions for managing Guillain Barre Syndrome.
Recent Developments
- In February 2025, Pune Municipal Corporation (PMC) in India launched extensive measures to combat the guillain-barre syndrome outbreak. PMC conducted health surveys amid increasing cases of GBS to ensure early diagnosis and treatment of patients of the guillain-barre syndrome.
- In December 2024, Hansa Biopharma announces positive full results from 15-HMedIdeS-09 Phase 2 study and comparative analysis of imlifidase in patients with the trial aims to evaluate the safety, tolerability, and efficacy of imlifidase when used in combination with the standard Intravenous Immunoglobulin (IVIG) treatment for GBS patients.
- In December 2023, QuidelOrtho received FDA 510(k) clearance for its Savanna multiplex molecular platform and the Savanna HSV 1+2/VZV PCR assay, enabling rapid detection and differentiation of HSV-1, HSV-2, and VZV from patient lesion samples.
- In October 2023, Annexon Inc announced that the European Medicine Agency (EMA) granted orphan drug designation to ANX005 for the treatment of Guillian-Bare Syndrome. This strategy is expected to enhance their outreach to untapped economies.
Report Coverage
The research report offers an in-depth analysis based on Gender, Age Group, End-User and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market will expand with rising awareness of early neurological disorder detection.
- Advanced biomarker-based assays will gain wider acceptance in clinical diagnostics.
- AI-driven tools will improve accuracy and reduce diagnostic turnaround times.
- Point-of-care diagnostic devices will see higher adoption in emergency settings.
- Emerging economies will invest more in healthcare infrastructure for neurology.
- Collaborations between hospitals, biotech firms, and research institutes will accelerate innovation.
- Personalized medicine will drive demand for precise and patient-specific diagnostic methods.
- Governments will increase funding for autoimmune and neurological research programs.
- Rehabilitation centers will play a greater role in long-term diagnostic follow-up.
- Global players will focus on expanding access in underserved regions.




