Market Overview
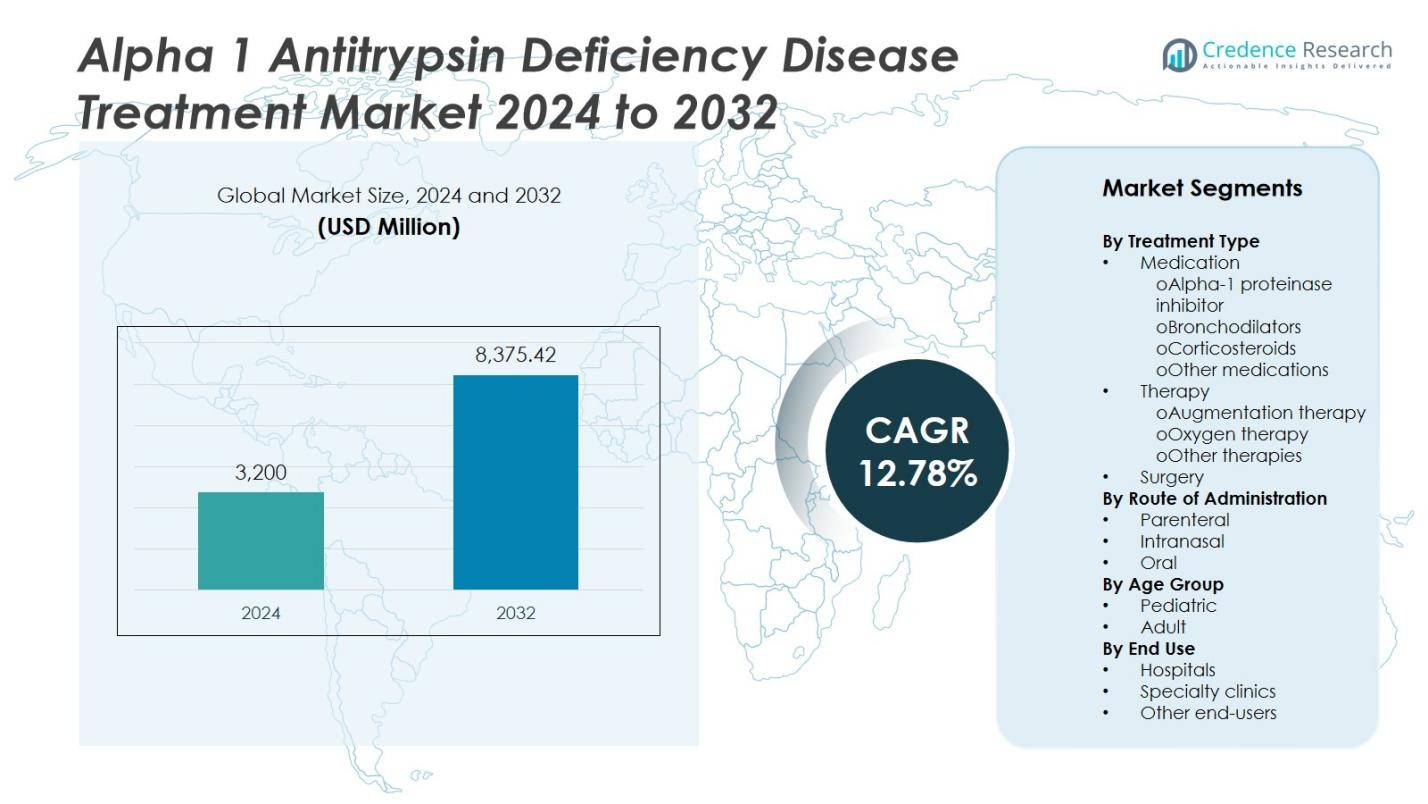
The Alpha-1 Antitrypsin Deficiency Disease Treatment Market size was valued at USD 3,200 million in 2024 and is anticipated to reach USD 8,375.42 million by 2032, expanding at a CAGR of 12.78% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Alpha-1 Antitrypsin Deficiency Disease Treatment Market Size 2024 |
USD 3,200 Million |
| Alpha-1 Antitrypsin Deficiency Disease Treatment Market, CAGR |
12.78% |
| Alpha-1 Antitrypsin Deficiency Disease Treatment Market Size 2032 |
USD 8,375.42 Million |
The Alpha-1 Antitrypsin Deficiency Disease Treatment Market is led by major players such as GlaxoSmithKline plc, Grifols S.A., CSL Behring, CHIESI Farmaceutici S.p.A., Arrowhead Pharmaceuticals, Intellia Therapeutics, Kamada Pharmaceuticals, and Epicrispr Biotechnologies. These companies focus on developing advanced protein replacement therapies and gene-based treatments to address the genetic root of the disorder. Strategic collaborations and clinical advancements in RNA interference and CRISPR technologies are expanding treatment efficacy and patient access. North America dominated the market with a 42% share in 2024, driven by strong healthcare infrastructure, favorable reimbursement frameworks, and early adoption of novel therapeutics, followed by Europe with 30% supported by regulatory incentives for rare disease drug development.
Market Insights
- The Alpha-1 Antitrypsin Deficiency Disease Treatment Market was valued at USD 3,200 million in 2024 and is projected to reach USD 8,375.42 million by 2032, growing at a CAGR of 12.78%.
- Rising diagnosis rates, government awareness programs, and growing adoption of gene and protein replacement therapies are key factors driving market expansion.
- Increasing focus on personalized medicine, CRISPR-based innovations, and clinical trials for long-acting therapies are major trends shaping the industry.
- The market is moderately consolidated, with key players such as GlaxoSmithKline, Grifols, CSL Behring, and Arrowhead Pharmaceuticals dominating through innovation and partnerships.
- North America leads with 42% share, followed by Europe at 30%, while Asia-Pacific is the fastest-growing region; among treatment types, the medication segment holds 45% share, driven by the dominance of alpha-1 proteinase inhibitor formulations.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Segmentation Analysis:
By Treatment Type
The medication segment dominates the Alpha-1 Antitrypsin Deficiency Disease Treatment Market, accounting for over 45% of total market share in 2024. Among medications, alpha-1 proteinase inhibitors remain the most prescribed due to their effectiveness in slowing lung damage progression. Bronchodilators and corticosteroids complement treatment by improving respiratory function and managing inflammation. The rise in diagnosed cases, growing physician preference for targeted therapies, and availability of advanced formulations have strengthened this segment’s dominance. Continuous R&D in protein replacement therapies further supports steady revenue growth within the medication category.
For instance, CSL Behring’s Zemaira product offers advanced packaging options that streamline preparation and reduce waste, supporting sustained patient care.
By Route of Administration
The parenteral route held the leading position in 2024 with around 60% market share, primarily driven by the widespread use of intravenous alpha-1 proteinase inhibitor infusions. This route ensures faster drug absorption and higher bioavailability, which is vital for treating severe deficiency cases. Increasing hospital-based treatments and improved infusion facilities have enhanced patient compliance. Meanwhile, intranasal and oral routes are gaining gradual traction due to ease of administration and ongoing clinical research into less invasive delivery systems that could broaden patient accessibility.
For instance, Grifols completed enrollment of the second cohort in its first-in-human trial for a 15% subcutaneous alpha-1 proteinase inhibitor formulation aimed at improving patient convenience and treatment flexibility.
By Age Group
The adult segment accounted for nearly 70% of the market share in 2024, reflecting the higher prevalence of Alpha-1 Antitrypsin Deficiency among adults aged 30–60 years. Early diagnosis programs, coupled with improved awareness of genetic testing, have expanded the adult treatment base. Additionally, lifestyle factors such as smoking and occupational exposure to pollutants increase disease severity in adults, driving greater demand for augmentation and inhalation therapies. Pediatric cases, though less frequent, are expected to see moderate growth with the advancement of gene-based treatments and newborn screening initiatives.
 Key Growth Drivers
Key Growth Drivers
Rising Diagnosis Rates and Awareness Programs
Increasing global awareness about Alpha-1 Antitrypsin Deficiency (AATD) has led to higher diagnosis rates and earlier intervention. Public health campaigns and genetic testing initiatives by healthcare authorities and organizations such as Alpha-1 Foundation are helping identify at-risk individuals sooner. Improved physician education and patient registries have also expanded treatment access. This growing diagnosis base directly boosts the demand for medications and augmentation therapies, making awareness initiatives a vital factor in driving long-term market expansion.
For instance, patient registries like the National Alpha-1 Antitrypsin Deficiency Registry in Ireland collect detailed medical data to improve clinical care and facilitate earlier access to treatments, supporting expanded treatment coverage.
Advancements in Gene and Protein Replacement Therapies
The development of next-generation therapies, including gene editing and protein replacement treatments, is significantly transforming AATD care. Companies like Intellia Therapeutics and Arrowhead Pharmaceuticals are advancing RNA interference and CRISPR-based solutions that target the genetic cause of the disease. These innovations promise more durable outcomes compared to traditional protein infusions. As clinical trials progress toward regulatory approval, the entry of gene-based products is expected to enhance treatment efficacy and accelerate market growth through the next decade.
For instance, Intellia Therapeutics has obtained UK regulatory clearance to start a Phase 1/2 clinical trial of NTLA-3001, an in vivo CRISPR-Cas9 gene insertion therapy designed to insert a functional copy of the SERPINA1 gene and restore normal AAT protein levels after a single dose.
Rising Healthcare Expenditure and Access to Specialty Care
Growing healthcare expenditure in developed and emerging economies supports better access to specialized AATD treatments. Governments and insurers are increasingly including proteinase inhibitor therapy under reimbursement frameworks, making it more affordable for patients. The expansion of specialized respiratory clinics and diagnostic centers also facilitates early treatment adoption. Coupled with technological advancements in drug manufacturing, these factors strengthen patient outcomes and fuel consistent demand for effective therapeutic options worldwide.
Key Trends and Opportunities
Expansion of Personalized and Precision Medicine Approaches
Personalized medicine is gaining traction in AATD management, focusing on genotype-specific therapies and individualized dosing. Advances in molecular diagnostics enable clinicians to tailor treatments based on patient genetics and disease progression. Pharmaceutical companies are investing in biomarker research to optimize therapy outcomes and reduce side effects. This trend aligns with the broader healthcare movement toward targeted interventions, offering a major opportunity for market players to develop patient-specific therapeutic models that improve efficacy and long-term disease control.
For instance, CRISPR/Cas9 gene editing techniques are under development to target specific mutations in the AAT gene, aiming for long-term therapeutic correction in AATD patients.
Growing Potential in Emerging Markets
Emerging economies in Asia-Pacific and Latin America present new opportunities due to improving healthcare infrastructure and diagnostic capabilities. Governments are prioritizing rare disease management programs, creating avenues for treatment expansion. Local partnerships and strategic collaborations with regional healthcare providers enable global firms to penetrate untapped markets. With increasing awareness campaigns and accessibility to advanced therapies, these regions are expected to witness faster adoption of AATD treatments, contributing significantly to overall market growth during the forecast period.
For instance, Biopas, a leading pharmaceutical company in Latin America, focuses on in-licensing and marketing cutting-edge specialty pharmaceuticals, including orphan drugs, which supports improved access to AATD treatments across Latin American countries through localized partnerships and tailored solutions.
Key Challenges
High Cost of Treatment and Limited Reimbursement
The high cost of proteinase inhibitor therapy and emerging gene-based treatments remains a major market restraint. Annual treatment expenses can exceed several thousand dollars, limiting affordability for patients without strong insurance support. Reimbursement policies vary across countries, and access to subsidized care is often restricted. This cost barrier discourages adherence and delays treatment initiation, particularly in low-income regions. Overcoming pricing and reimbursement challenges is essential for expanding the patient base and ensuring sustainable market development.
Limited Awareness in Developing Regions
Despite global progress, awareness of Alpha-1 Antitrypsin Deficiency remains low in developing countries. Many patients remain undiagnosed due to inadequate genetic testing and misdiagnosis as chronic obstructive pulmonary disease (COPD). The absence of national screening programs further exacerbates underdiagnosis rates. Lack of clinical expertise and limited access to specialized healthcare centers hinder early detection and intervention. Addressing these educational and infrastructural gaps through coordinated government and industry initiatives is crucial to achieving equitable global treatment access.
Regional Analysis
North America
North America dominates the Alpha-1 Antitrypsin Deficiency Disease Treatment Market, holding a market share of 42% in 2024. The region benefits from strong healthcare infrastructure, early diagnosis programs, and extensive awareness initiatives led by organizations such as the Alpha-1 Foundation. The United States drives most of the demand due to high treatment adoption, government funding for rare diseases, and the presence of key players like Arrowhead Pharmaceuticals and Grifols S.A. Favorable reimbursement policies and advanced biopharmaceutical research continue to reinforce the region’s leadership throughout the forecast period.
Europe
Europe accounts for a market share of 30% in 2024, supported by an established regulatory framework and access to advanced protein replacement therapies. Countries such as Germany, the United Kingdom, and France lead regional adoption, driven by high awareness levels and expanding genetic testing programs. The European Medicines Agency’s support for orphan drug development further accelerates market growth. Partnerships between hospitals and pharmaceutical firms enhance accessibility, while national healthcare systems play a critical role in reimbursement and patient care. Continued innovation in augmentation therapy sustains Europe’s strong position.
Asia-Pacific
The Asia-Pacific region represents 18% of the market share in 2024 and is projected to register the fastest growth through 2032. Rising healthcare expenditure, expanding clinical research, and improved diagnostic capabilities in countries like China, Japan, and India drive regional momentum. Increasing awareness of rare genetic disorders and investments by global pharmaceutical firms in regional collaborations are boosting treatment access. Government initiatives supporting early screening and healthcare modernization also contribute to higher adoption rates. The growing patient pool and technological advancements make Asia-Pacific a key emerging growth hub.
Latin America
Latin America holds a market share of 6% in 2024, driven by gradual improvements in diagnostic infrastructure and access to imported therapies. Brazil and Mexico are the leading contributors, benefiting from the presence of specialized respiratory centers and government-backed healthcare programs. While awareness remains limited compared to developed regions, ongoing training initiatives for healthcare professionals and partnerships with international pharmaceutical companies are helping increase early detection rates. The region shows potential for growth as reimbursement systems and regulatory approvals evolve to support rare disease treatments.
Middle East and Africa
The Middle East and Africa together account for a market share of 4% in 2024, reflecting developing healthcare systems and limited diagnostic capabilities. Gulf Cooperation Council (GCC) countries, particularly Saudi Arabia and the UAE, are investing in genetic research and rare disease treatment centers. However, low awareness and inadequate access to protein replacement therapies restrict widespread adoption. Efforts by international health organizations to improve education and infrastructure are slowly expanding treatment availability. Increasing government focus on healthcare diversification offers gradual improvement prospects in the coming years.
Market Segmentations:
By Treatment Type
- Medication
- Alpha-1 proteinase inhibitor
- Bronchodilators
- Corticosteroids
- Other medications
- Therapy
-
- Augmentation therapy
- Oxygen therapy
- Other therapies
By Route of Administration
- Parenteral
- Intranasal
- Oral
By Age Group
By End Use
- Hospitals
- Specialty clinics
- Other end-users
By Region
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Competitive Landscape
The competitive landscape of the Alpha-1 Antitrypsin Deficiency Disease Treatment Market includes key players such as GlaxoSmithKline plc, Grifols S.A., CSL Behring, CHIESI Farmaceutici S.p.A., Arrowhead Pharmaceuticals, Intellia Therapeutics, Kamada Pharmaceuticals, Epicrispr Biotechnologies, Mayo Foundation for Medical Education and Research, and National Jewish Health. These companies focus on developing advanced protein replacement and gene therapy solutions to improve treatment efficacy. Strategic partnerships, clinical trial expansions, and investments in CRISPR and RNA interference technologies strengthen their market presence. Grifols and CSL Behring lead the augmentation therapy segment through robust distribution networks and production capabilities. Meanwhile, emerging biopharma firms such as Arrowhead and Intellia are advancing gene-based innovations targeting disease correction at the molecular level. Collaborations with research institutions and universities enhance product development pipelines, while government incentives for orphan drug development continue to attract investment, ensuring sustained competition and innovation within the market.
Key Player Analysis
- GlaxoSmithKline plc
- Kamada Pharmaceuticals
- Epicrispr Biotechnologies, Inc.
- Grifols S.A.
- Arrowhead Pharmaceuticals, Inc.
- CHIESI Farmaceutici S.p.A.
- Intellia Therapeutics, Inc.
- CSL Behring
- Mayo Foundation for Medical Education and Research
- National Jewish Health
Recent Developments
- In July 2024, GSK announced the ongoing clinical development of GSK-5462688, an investigational treatment for alpha-1 antitrypsin deficiency, which is currently in Phase II clinical trials. GSK-5462688 is designed to address the genetic disorder alpha-1 antitrypsin deficiency, which can lead to severe lung and liver disease. The outcome of this trial is anticipated to significantly impact GSK’s position in the treatment landscape for genetic disorders.
- In July 2024, Intellia Therapeutics received authorization to initiate a Phase 1/2 clinical trial for NTLA-3001, a groundbreaking gene editing treatment targeting alpha-1 antitrypsin deficiency. NTLA-3001 represents Intellia’s first wholly owned CRISPR-based in vivo targeted gene insertion candidate to progress into clinical trials. This strategy is expected to add value to the company’s business portfolio.
- On March 18 2025, Prime Medicine disclosed a gene-editing program aimed at correcting the Pi*Z mutation in the SERPINA1 gene for AATD treatment.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Report Coverage
The research report offers an in-depth analysis based on Treatment Type, Route of Administration, End Use and Region. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market is expected to witness steady growth driven by advancements in gene therapy.
- Increasing government support for rare disease treatment will enhance patient accessibility.
- New entrants focusing on CRISPR and RNA technologies will intensify competition.
- Partnerships between biotech firms and research institutions will accelerate therapy innovation.
- Expanding diagnostic programs will help identify more patients for early intervention.
- Rising healthcare investment in emerging economies will unlock untapped opportunities.
- Broader insurance coverage for protein replacement therapy will boost treatment adoption.
- Continuous improvement in manufacturing processes will lower therapy costs over time.
- Growing patient awareness and education initiatives will expand treatment acceptance.
- Ongoing clinical trials for long-acting and curative therapies will redefine the market landscape.

 Key Growth Drivers
Key Growth Drivers

