Market Overview:
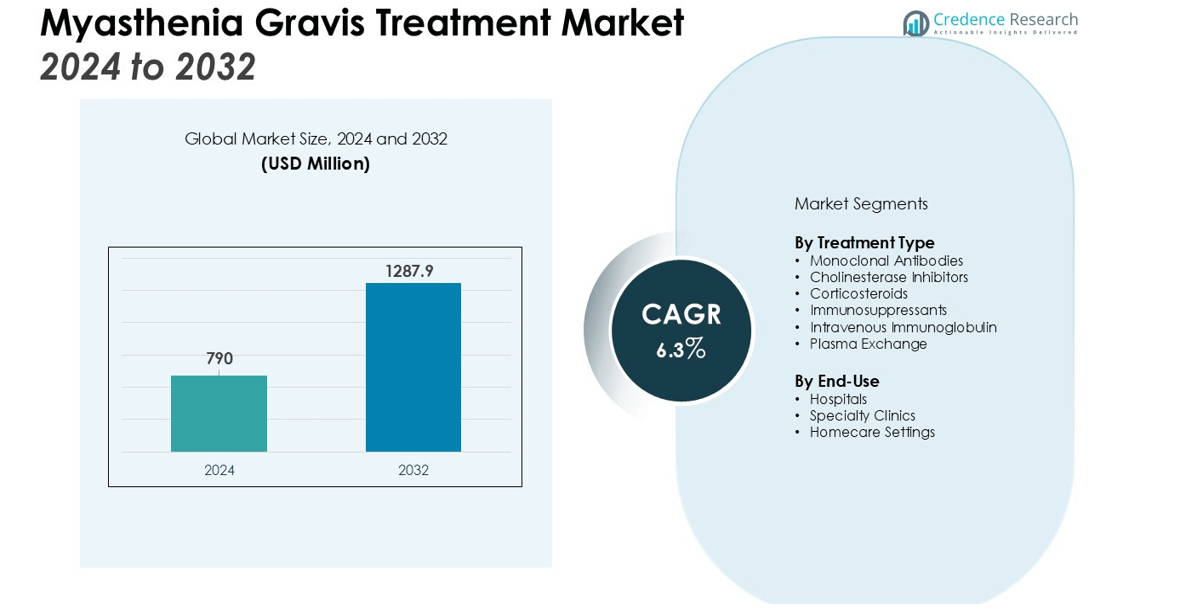
The Myasthenia Gravis Treatment Market size was valued at USD 790 million in 2024 and is anticipated to reach USD 1287.9 million by 2032, at a CAGR of 6.3% during the forecast period (2024-2032).
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Myasthenia Gravis Treatment Market Size 2024 |
USD 790 million |
| Myasthenia Gravis Treatment Market, CAGR |
6.3% |
| Myasthenia Gravis Treatment Market Size 2032 |
USD 1287.9 million |
Key growth drivers include improved diagnostic accuracy via antibody testing and electrophysiological tools, fueling earlier intervention. Breakthrough therapies—such as monoclonal antibodies, targeted immunotherapies, and complement inhibitors—address unmet medical needs more effectively. Enhanced reimbursement frameworks and expanding insurance coverage in developed markets support patient access to novel treatments. Additionally, ongoing clinical trials and rising research investment continue to push treatment innovation forward, strengthening growth momentum.
North America dominates with strong healthcare infrastructure, high awareness, and early uptake of advanced therapies. Europe follows closely, benefiting from unified regulatory standards and reimbursement systems. Asia-Pacific is the fastest-growing region, as improving healthcare access, growing medical infrastructure, and increasing awareness drive demand in China, India, and Southeast Asia. Emerging markets in Latin America and the Middle East also see gradual adoption, thanks to expanding healthcare budgets and growing patient diagnosis rates. The global market outlook is further reinforced by increasing collaborations between pharmaceutical companies and research institutions to accelerate therapy development.
Market Insights:
- The Myasthenia Gravis Treatment Market was valued at USD 790 million and is projected to reach USD 1287.9 million by 2032, reflecting a CAGR of 6.3%.
- Advancements in antibody testing and electrophysiological tools enable earlier detection, supporting timely interventions and better outcomes.
- Innovative therapies such as monoclonal antibodies, complement inhibitors, and targeted immunotherapies are redefining treatment standards.
- Insurance coverage and reimbursement frameworks in developed regions reduce financial barriers to high-cost therapies.
- North America leads with 42% share, driven by strong healthcare infrastructure and advanced therapy adoption.
- Europe holds 30% share, supported by favorable reimbursement systems and robust clinical trial activity.
- Asia-Pacific, with 18% share, is the fastest-growing region due to expanding healthcare access, rising awareness, and government support.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers:
Advancements in Diagnostic Tools and Early Detection
The Myasthenia Gravis Treatment Market benefits from significant progress in diagnostic technologies. Antibody testing and electrophysiological tools have improved the accuracy of detection, allowing earlier intervention and timely treatment planning. Early diagnosis ensures that patients can access targeted therapies sooner, reducing the progression of symptoms. It also supports physicians in tailoring therapies more effectively to patient needs, enhancing treatment outcomes and overall disease management.
Emergence of Breakthrough Therapeutic Options
The market experiences strong growth from the development of advanced therapies such as monoclonal antibodies, complement inhibitors, and targeted immunotherapies. These treatments address unmet needs that traditional medications cannot manage effectively. It has allowed patients with severe or refractory conditions to gain access to improved quality of care. The introduction of innovative drug classes demonstrates the shift toward precision medicine, where therapies target specific disease mechanisms rather than providing broad immunosuppression.
- For instance, Biogen’s monoclonal antibody drug Aduhelm was approved by the FDA in 2021 for Alzheimer’s disease, with clinical studies demonstrating a reduction in amyloid beta plaques in the brain of treated patients.
Supportive Reimbursement Policies and Insurance Coverage
Reimbursement frameworks play a critical role in the expansion of the Myasthenia Gravis Treatment Market. Governments and private insurers have expanded coverage to include novel and high-cost therapies, encouraging patient access to advanced treatments. This financial support reduces barriers to adoption, particularly in developed economies. It also motivates pharmaceutical companies to bring innovative therapies to market, knowing patients can access them without overwhelming financial burdens.
- For instance, Johnson & Johnson’s IMAAVY (nipocalimab) received FDA approval for generalized Myasthenia Gravis treatment and offers a support program that enables commercially insured patients to receive treatment with near-zero out-of-pocket costs per infusion.
Research Investments and Clinical Development Initiatives
Ongoing research programs continue to strengthen the future of treatment for this rare disease. Pharmaceutical companies and academic institutions are investing in clinical trials that test new drug candidates and optimize existing therapies. The Myasthenia Gravis Treatment Market benefits from these efforts through a growing pipeline of options that promise better safety and efficacy profiles. It positions the industry for long-term growth while offering hope for patients seeking more sustainable disease management solutions.
Market Trends:
Rising Focus on Targeted and Precision-Based Therapies
The Myasthenia Gravis Treatment Market is witnessing a strong shift toward precision-based therapies that address underlying disease mechanisms. Pharmaceutical companies are prioritizing monoclonal antibodies, complement inhibitors, and Fc receptor modulators, which offer more effective outcomes compared to conventional immunosuppressants. It reflects the growing demand for treatments with fewer side effects and improved safety profiles. Patients are increasingly gaining access to therapies designed to control specific immune pathways, which is reshaping clinical practice. Clinical guidelines are also evolving to incorporate these targeted options, creating wider acceptance among healthcare professionals. This trend is supported by the steady approval of new biologics, reinforcing the role of innovation in treatment standards.
- For instance, in a real-life study involving 119 myasthenia gravis patients treated with eculizumab, 70 patients showed clinical improvement at 24 months, with the MG-ADL score decreasing from 8 to 4.7 after treatment.
Growing Role of Digital Health and Patient-Centric Approaches
The market is also marked by the adoption of digital health solutions that enhance patient monitoring and disease management. Telemedicine, electronic health records, and wearable devices enable real-time tracking of symptoms and treatment responses. It has expanded opportunities for physicians to make data-driven decisions that improve care delivery. The Myasthenia Gravis Treatment Market benefits from these tools by fostering better adherence and early intervention strategies. Patient-centric approaches are becoming central to clinical programs, with a focus on personalized dosing and long-term therapy management. Pharmaceutical companies are collaborating with healthcare providers to integrate digital tools into standard treatment models. This convergence of technology and care is setting new benchmarks for patient engagement and treatment outcomes.
- For instance, Epic Systems supports over 250 million patients worldwide through its electronic health record system, highlighting its extensive role in integrating patient health data for improved care delivery.
Market Challenges Analysis:
High Treatment Costs and Limited Accessibility
The Myasthenia Gravis Treatment Market faces challenges linked to the high cost of advanced therapies. Monoclonal antibodies and complement inhibitors offer clinical benefits but remain expensive, limiting patient access in many regions. Insurance coverage and reimbursement frameworks reduce the burden in developed markets, yet gaps persist in emerging economies. It creates inequalities in treatment availability, restricting widespread adoption of innovative options. Smaller healthcare systems often struggle to allocate resources for rare disease management. This barrier underscores the need for cost-effective solutions and broader affordability measures.
Clinical Complexity and Limited Awareness
The market also encounters obstacles from the clinical complexity of the disease and insufficient awareness among patients and healthcare providers. Myasthenia gravis symptoms can mimic other neurological conditions, leading to delayed or incorrect diagnoses. It hinders timely treatment and may worsen patient outcomes. Limited knowledge in underserved regions further reduces the effectiveness of available therapies. Clinical trial recruitment is another concern, as small patient pools make it difficult to conduct large-scale studies. These challenges continue to influence how quickly new therapies gain acceptance in the Myasthenia Gravis Treatment Market.
Market Opportunities:
Expansion of Biologic and Targeted Therapy Portfolio
The Myasthenia Gravis Treatment Market presents opportunities through the growing development of biologics and targeted therapies. Pharmaceutical companies are advancing monoclonal antibodies, complement inhibitors, and Fc receptor modulators that offer improved efficacy and safety. It creates room for expansion into patient groups not adequately managed by conventional immunosuppressants. Rising regulatory approvals in multiple regions further strengthen the commercial potential of these therapies. Increasing investment in research pipelines ensures a steady flow of innovative drugs. Companies focusing on rare disease treatment gain strategic advantage by addressing high unmet medical needs.
Growth Potential in Emerging Healthcare Markets
Emerging economies provide a strong growth platform for the Myasthenia Gravis Treatment Market due to improving healthcare infrastructure and rising awareness. Governments are increasing budgets for rare disease management, creating opportunities for wider adoption of novel therapies. It highlights the importance of partnerships between global pharmaceutical firms and local healthcare providers. Patient advocacy groups are also driving awareness, ensuring earlier diagnosis and treatment access. Expanding insurance coverage and evolving reimbursement models support affordability in these regions. The shift toward patient-centric healthcare systems is opening new pathways for sustainable market growth.
Market Segmentation Analysis:
By Treatment Type
The Myasthenia Gravis Treatment Market is segmented into monoclonal antibodies, cholinesterase inhibitors, corticosteroids, immunosuppressants, intravenous immunoglobulin, and plasma exchange. Monoclonal antibodies are gaining strong momentum due to their targeted action and effectiveness in refractory cases. Cholinesterase inhibitors remain widely prescribed as first-line therapies for symptomatic management. Corticosteroids and immunosuppressants continue to play a role in controlling immune responses, though long-term use raises safety concerns. Intravenous immunoglobulin and plasma exchange are applied in acute situations, offering rapid relief during severe exacerbations. It highlights the balance between established therapies and advanced biologics shaping treatment adoption.
- For instance, in a cohort of 200 patients treated with Zilucoplan, 33 patients achieved corticosteroid dose reduction or discontinuation, with a mean dose decrease of 15.5 mg after 120 weeks of treatment.
By End-Use
The market is divided into hospitals, specialty clinics, and homecare settings. Hospitals dominate due to the availability of specialized neurology departments and access to advanced therapies. Specialty clinics are expanding their role by offering personalized treatment approaches and closer patient monitoring. It demonstrates strong demand for outpatient care, particularly where long-term disease management is required. Homecare settings are emerging gradually, supported by improved drug delivery methods and patient preference for comfort-based management. The Myasthenia Gravis Treatment Market reflects a shift toward integrated healthcare models that combine institutional expertise with patient-centric approaches.
- For instance, Cleveland Clinic performed 320,000 surgeries and procedures in 2024, demonstrating its extensive capacity in neurological care.
Segmentations:
By Treatment Type
- Monoclonal Antibodies
- Cholinesterase Inhibitors
- Corticosteroids
- Immunosuppressants
- Intravenous Immunoglobulin
- Plasma Exchange
By End-Use
- Hospitals
- Specialty Clinics
- Homecare Settings
By Region
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis:
Strong Market Position in North America
North America accounted for 42% share of the Myasthenia Gravis Treatment Market. The region benefits from advanced healthcare infrastructure, high awareness levels, and strong reimbursement frameworks. It continues to attract major pharmaceutical companies that focus on innovation and regulatory approvals. High diagnosis rates and the presence of specialized neurology centers further support growth. The United States remains the dominant contributor, backed by strong funding and advanced therapeutic availability.
Steady Growth and Supportive Systems in Europe
Europe held 30% share of the Myasthenia Gravis Treatment Market. Favorable reimbursement policies across major economies ensure patient access to high-cost therapies. It is also a hub for clinical trials, with collaborations between pharmaceutical companies and research institutions driving treatment innovation. Countries such as Germany, France, and the United Kingdom lead regional adoption. Growing investments in rare disease management strengthen long-term growth prospects. Increasing physician awareness and patient advocacy further expand treatment uptake.
Rapid Expansion in Asia-Pacific and Emerging Economies
Asia-Pacific represented 18% share of the Myasthenia Gravis Treatment Market. Rising awareness in China, India, and Southeast Asia contributes to higher diagnosis rates and treatment adoption. It benefits from government-led initiatives to enhance rare disease care and broaden insurance coverage. Pharmaceutical companies are forming alliances with regional players to strengthen distribution networks. Latin America and the Middle East are also experiencing gradual uptake, supported by expanding healthcare budgets. These emerging markets provide long-term opportunities for global players to increase their footprint.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
Competitive Analysis:
The Myasthenia Gravis Treatment Market is highly competitive, with global pharmaceutical companies focusing on innovation and strategic growth. Leading players emphasize research and development to advance monoclonal antibodies, complement inhibitors, and targeted immunotherapies. It drives strong differentiation in treatment options and positions companies to capture demand from patients with unmet medical needs. Strategic alliances, mergers, and acquisitions are common, enabling firms to expand portfolios and strengthen geographic reach. Companies also invest in clinical trials to secure regulatory approvals and build credibility in rare disease care. Competitive intensity is reinforced by increasing collaborations with academic institutions and healthcare providers, ensuring a steady pipeline of advanced therapies. The market reflects a balance between established players with strong distribution networks and emerging firms introducing niche solutions. It continues to evolve toward precision medicine, where innovation and affordability will define long-term competitive positioning.
Recent Developments:
- In July 2025, Alexion Pharmaceuticals entered into a license agreement with JCR Pharmaceuticals to use proprietary JUST-AAV capsid technology in gene therapy development for rare diseases.
- In August 2025, Pfizer and BioNTech’s COVID-19 vaccine COMIRNATY received FDA approval for an updated formulation targeting the Omicron LP.8.1 subvariant for adults 65+ and high-risk groups.
Report Coverage:
The research report offers an in-depth analysis based on Treatment Type, End-Use and Region. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook:
- The Myasthenia Gravis Treatment Market will expand with wider adoption of biologics and targeted therapies.
- It will see growing emphasis on precision medicine, focusing on therapies that address immune pathways.
- Pharmaceutical companies will invest more in clinical trials to strengthen pipelines and secure new approvals.
- The market will benefit from rising patient awareness and improved diagnostic capabilities across all regions.
- Hospitals and specialty clinics will continue to dominate care delivery, driven by advanced treatment availability.
- It will experience increased integration of digital health tools to support patient monitoring and adherence.
- Emerging economies will drive growth with expanding healthcare infrastructure and government-led rare disease programs.
- Strategic collaborations between pharmaceutical firms and research institutions will accelerate therapy development and distribution.
- The market will face ongoing pressure to address affordability through supportive reimbursement and pricing models.
- It will evolve toward patient-centric care models, offering sustainable solutions and improving long-term treatment outcomes.




