Market Overview
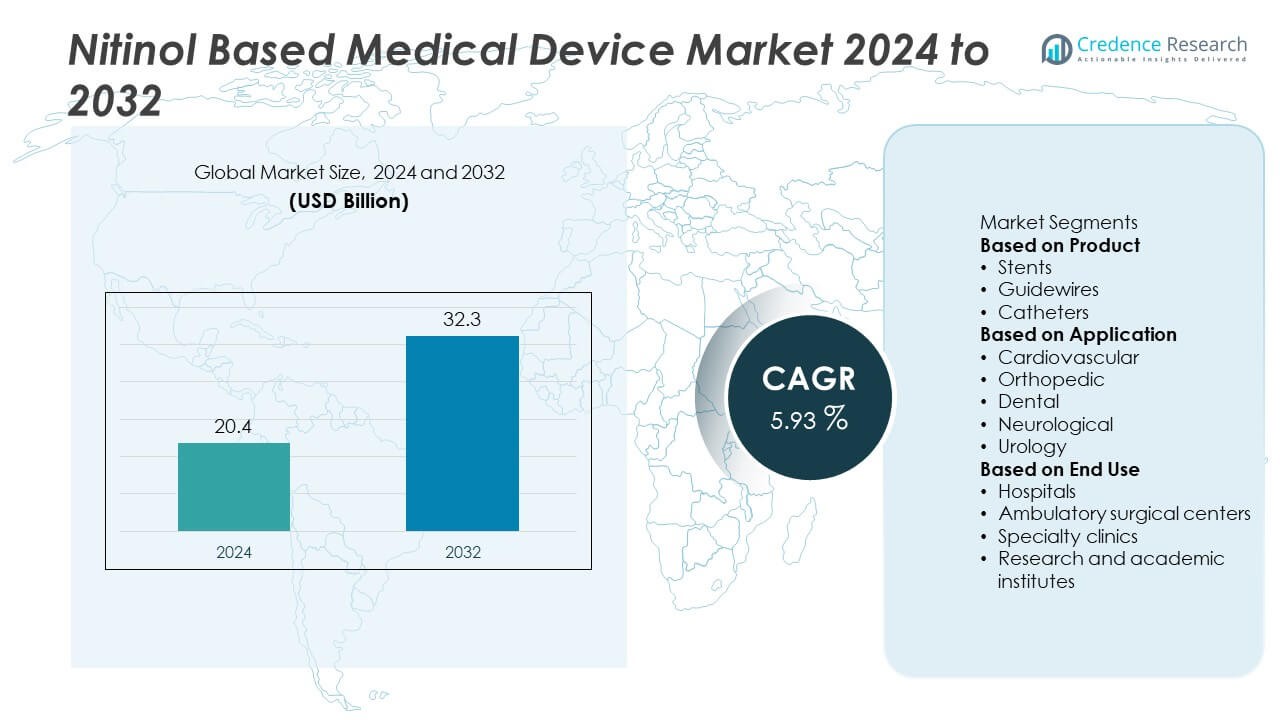
The Nitinol Based Medical Device Market size was valued at USD 20.4 billion in 2024 and is projected to reach USD 32.3 billion by 2032, expanding at a CAGR of 5.93% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2024 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Nitinol Based Medical Device Market Size 2024 |
USD 20.4 Billion |
| Nitinol Based Medical Device Market, CAGR |
5.93% |
| Nitinol Based Medical Device Market Size 2032 |
USD 32.3 Billion |
The Nitinol Based Medical Device Market grows through rising demand for minimally invasive procedures, supported by the superior flexibility, durability, and shape memory properties of Nitinol. Cardiovascular, orthopedic, and neurovascular applications remain primary drivers, with hospitals adopting advanced stents, guidewires, and implants to improve patient outcomes.
The Nitinol Based Medical Device Market demonstrates strong geographical presence across North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. North America leads adoption due to advanced healthcare infrastructure and high demand for minimally invasive procedures. Europe follows with strong clinical uptake in cardiovascular and orthopedic segments, supported by innovation hubs and strict regulatory standards. Asia Pacific emerges as the fastest-growing region, fueled by expanding healthcare investments and large patient populations in countries such as China, Japan, and India. Latin America and the Middle East & Africa show gradual growth supported by rising awareness and healthcare reforms. Key players driving the market include Abbott Laboratories, Boston Scientific, and Biotronik, known for their broad product portfolios and continuous innovation. Companies such as B. Braun also expand their presence through advanced stent systems and collaborations, reinforcing competitiveness across multiple therapeutic areas.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The Nitinol Based Medical Device Market was valued at USD 20.4 billion in 2024 and is projected to reach USD 32.3 billion by 2032, growing at a CAGR of 5.93% during the forecast period.
- Growing demand for minimally invasive surgeries drives adoption, as Nitinol’s shape memory and flexibility improve surgical outcomes. Its role in cardiovascular, orthopedic, and neurovascular procedures strengthens market expansion.
- Trends highlight rising integration with robotics, imaging systems, and digital navigation tools, which enhance surgical precision and reduce complications. Personalized implants and surface coating innovations further expand adoption.
- Competition intensifies as global players such as Abbott Laboratories, Boston Scientific, and Biotronik advance product portfolios, while companies like B. Braun and Cook Medical invest in new stent and guidewire technologies. Partnerships and R&D programs remain central strategies for maintaining competitiveness.
- High production costs and complex manufacturing processes pose restraints, limiting scalability and affordability for smaller healthcare facilities. Regulatory challenges and biocompatibility concerns, particularly related to nickel release, also affect adoption rates.
- North America leads with strong technological adoption and robust healthcare infrastructure, while Europe maintains steady growth supported by clinical innovation and an aging population. Asia Pacific records the fastest growth, driven by expanding healthcare systems and large patient pools in China, Japan, and India. Latin America and the Middle East & Africa display gradual yet consistent expansion through healthcare reforms and rising awareness.
- Continuous R&D investment in next-generation devices, sustainability-focused innovation, and emerging opportunities in developing economies create a favorable long-term outlook for the market.
Market Drivers
Growing Use of Nitinol in Minimally Invasive Surgical Procedures
The Nitinol Based Medical Device Market benefits from the rising demand for minimally invasive surgeries. Surgeons prefer Nitinol-based stents, guidewires, and catheters due to their flexibility and durability. These properties allow devices to navigate complex vascular pathways with reduced trauma to patients. Hospitals invest in advanced Nitinol devices to shorten recovery times and lower complication risks. Clinical adoption grows steadily across cardiovascular and neurovascular specialties where precision is critical. It continues to reinforce the role of Nitinol as a material of choice in next-generation surgical devices.
- For instance, Abbott’s Supera peripheral stent delivered zero fractures at its one-year follow-up. This high fracture resistance is attributed to its flexible, interwoven nitinol wire design, which mimics artery movement.
Rising Demand for Cardiovascular and Orthopedic Implants
Cardiovascular diseases remain a major driver for growth in the Nitinol Based Medical Device Market. Nitinol stents and filters provide high fatigue resistance, ensuring reliable long-term performance. Orthopedic implants that use Nitinol demonstrate strong shape memory properties, helping improve bone fixation and joint stability. Patient preference for durable implants strengthens market demand across aging populations. Healthcare providers highlight clinical data that confirms improved outcomes with Nitinol-based implants. It ensures consistent expansion in both high-income and emerging healthcare systems.
Expanding Role in Robotic-Assisted and Image-Guided Surgeries
The Nitinol Based Medical Device Market advances with the integration of robotics and image-guided procedures. Robotic-assisted surgeries require highly flexible instruments, making Nitinol-based devices critical to precision outcomes. Image-guided platforms rely on shape memory features to maintain accuracy under complex conditions. Hospitals adopt these devices to improve efficiency and reduce human error during surgery. The synergy of robotics and Nitinol expands surgical possibilities in complex procedures. It supports the trend of technology-driven healthcare transformation worldwide.
- For instance, Intuitive Surgical integrates Nitinol’s superelastic properties into the instruments for its da Vinci Surgical System. One surgical robot arm incorporates over 120 discrete Nitinol components, allowing for precise, flexible movements that would be impossible with rigid materials. This enables surgeons to perform intricate tasks with enhanced dexterity during minimally invasive procedures.
Strong Research and Development Investment in Next-Generation Devices
The Nitinol Based Medical Device Market grows through sustained research initiatives by global manufacturers. Companies allocate resources to enhance biocompatibility, corrosion resistance, and fatigue life. R&D pipelines focus on innovative applications in vascular closure systems, orthopedic implants, and structural heart devices. Industry collaborations with research institutes strengthen the pace of clinical adoption. Regulatory approvals support faster market entry for breakthrough Nitinol-based products. It ensures a continuous cycle of innovation that sustains long-term competitiveness.
Market Trends
Integration of Nitinol Devices with Advanced Imaging and Navigation Technologies
The Nitinol Based Medical Device Market witnesses growing integration with advanced imaging and navigation platforms. Surgeons rely on fluoroscopy, MRI, and CT-guided systems to improve accuracy during implantation. Nitinol devices complement these technologies with their shape memory and superelasticity, enhancing surgical outcomes. Hospitals expand investments in hybrid operating rooms equipped with advanced imaging support. This trend strengthens the precision and safety of complex cardiovascular and neurovascular procedures. It reflects a broader shift toward technology-driven healthcare solutions that improve patient outcomes.
- For instance, in the ILUMIEN IV clinical study, OCT-guided stent placement enabled physicians to achieve a greater minimal stent area in complex cases compared to angiography guidance.
Increasing Use of Nitinol in Next-Generation Structural Heart Devices
The Nitinol Based Medical Device Market gains momentum from its expanding role in structural heart therapies. Devices such as transcatheter aortic valve replacement systems and occluders use Nitinol for durability and flexibility. Clinical data confirms strong long-term reliability in patients with high-risk cardiac conditions. Medical centers adopt these technologies to address rising cases of valvular heart diseases. The unique mechanical properties of Nitinol allow for minimally invasive delivery and precise deployment. It ensures continued preference for Nitinol in life-saving cardiovascular interventions.
- For instance, the ACURATE neo2 transcatheter aortic valve system showed a 0.8% all-cause mortality rate at 30 days in a post‑market study of 250 high‑risk patients.
Expansion of Personalized and Patient-Specific Nitinol Implants
The Nitinol Based Medical Device Market experiences growth with the trend toward personalized implants. Advances in 3D printing and digital modeling enable customization of devices for individual patients. Orthopedic and dental applications adopt Nitinol implants designed to match patient anatomy. Hospitals report improved patient satisfaction and shorter recovery periods with tailored solutions. This trend supports precision medicine initiatives across global healthcare systems. It drives strong adoption of Nitinol devices in applications requiring custom-fit performance.
Sustainability and Biocompatibility Driving Innovation in Device Design
The Nitinol Based Medical Device Market evolves with focus on sustainability and biocompatibility. Manufacturers work to reduce nickel ion release while enhancing corrosion resistance. Regulatory bodies emphasize stricter compliance with safety and environmental standards. Companies innovate coatings and surface treatments that extend device lifespan and improve compatibility. Research pipelines explore alloy modifications that further minimize risks of adverse reactions. It ensures that Nitinol remains central to the development of safer and more sustainable medical devices.
Market Challenges Analysis
High Production Costs and Complex Manufacturing Processes
The Nitinol Based Medical Device Market faces challenges due to the high cost of production and complex manufacturing requirements. Nitinol’s unique shape memory and superelastic properties demand advanced processing techniques such as precision melting and specialized surface treatments. These processes increase expenses and limit the scalability of production for mass-market adoption. Small and mid-sized manufacturers struggle to meet cost efficiency standards while maintaining product quality. Healthcare providers in price-sensitive markets find Nitinol devices less accessible compared to alternatives. It creates pressure on producers to invest in cost reduction strategies without compromising performance.
Regulatory Barriers and Biocompatibility Concerns
The Nitinol Based Medical Device Market encounters regulatory hurdles and biocompatibility issues that slow adoption. Strict approval processes across regions require extensive testing for safety, fatigue resistance, and long-term performance. Delays in regulatory clearance extend product launch timelines and limit revenue growth opportunities. Concerns about nickel ion release raise questions on potential allergic reactions in sensitive patients. Manufacturers must balance innovation with compliance to meet evolving standards of patient safety. It places a continuous burden on companies to improve material purity, coatings, and testing protocols to satisfy regulators and healthcare providers.
Market Opportunities
Expanding Applications in Emerging Healthcare Markets
The Nitinol Based Medical Device Market holds significant opportunities in expanding healthcare infrastructure across emerging economies. Growing investments in hospitals and specialty clinics increase demand for advanced cardiovascular, orthopedic, and neurovascular solutions. Rising healthcare access in Asia Pacific, Latin America, and the Middle East creates strong adoption potential. Nitinol’s durability and flexibility make it highly suitable for regions where long-term device reliability is critical. Companies entering these markets benefit from untapped patient populations with rising income levels. It positions Nitinol devices as valuable solutions for both developed and developing healthcare systems.
Advancements in Next-Generation Therapeutic Applications
The Nitinol Based Medical Device Market gains opportunities from breakthroughs in therapeutic applications beyond traditional implants. Research into Nitinol-based drug delivery systems, minimally invasive vascular closure devices, and innovative orthopedic solutions strengthens product pipelines. Integration with robotics and digital health technologies enhances the scope of advanced surgical procedures. Industry collaborations with academic institutions accelerate innovation and clinical validation. Growing focus on personalized medicine further supports adoption of custom-designed Nitinol implants. It creates a pathway for sustained growth through diverse medical applications and advanced healthcare technologies.
Market Segmentation Analysis:
By Product
The Nitinol Based Medical Device Market demonstrates strong segmentation by product, with stents holding a leading share due to widespread cardiovascular applications. Guidewires and catheters follow, driven by their flexibility and reliability in minimally invasive procedures. Orthopedic implants using Nitinol alloys show consistent demand because of their shape memory and biocompatibility properties. Filters, staples, and vascular closure devices also gain traction as surgeons adopt advanced materials to improve safety and patient outcomes. Innovations in coatings and surface treatments further expand opportunities across product categories. It continues to diversify across multiple device types, reflecting ongoing advancements in medical technology.
- For instance, the Boston Scientific WATCHMAN FLX device achieved a 97.5% implant success rate among 97,185 patients in a real-world setting. This study demonstrated safety and effectiveness across diverse anatomies, showing outcomes comparable to clinical trials.
By Application
The Nitinol Based Medical Device Market expands across cardiovascular, orthopedic, dental, and neurovascular applications. Cardiovascular devices dominate, with Nitinol stents, occluders, and valves proving critical in managing chronic heart conditions. Orthopedic uses grow steadily as implants demonstrate superior flexibility and fatigue resistance compared to conventional materials. Dental applications gain importance through precision implants and orthodontic wires designed for durability and patient comfort. Neurovascular devices capture increasing interest due to the material’s ability to navigate delicate vascular pathways. It secures growth by meeting diverse clinical needs across high-demand medical fields.
- For instance, in the ILUMIEN IV imaging trial, OCT-guided stent implantation delivered a minimal stent area of 5.72 mm², compared to 5.36 mm² under angiography guidance.
By End Use
The Nitinol Based Medical Device Market divides into hospitals, specialty clinics, and ambulatory surgical centers. Hospitals lead adoption as they invest in advanced surgical infrastructure and high-volume cardiovascular and orthopedic procedures. Specialty clinics emerge as key users, especially in interventional cardiology and dental care. Ambulatory surgical centers record growing demand because of their focus on minimally invasive outpatient procedures. Rising patient preference for faster recovery and cost-effective care enhances adoption outside traditional hospital settings. It highlights a shift toward decentralized healthcare delivery, ensuring wider accessibility of Nitinol-based solutions.
Segments:
Based on Product
- Stents
- Guidewires
- Catheters
Based on Application
- Cardiovascular
- Orthopedic
- Dental
- Neurological
- Urology
Based on End Use
- Hospitals
- Ambulatory surgical centers
- Specialty clinics
- Research and academic institutes
Based on the Geography:
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis
North America
North America accounts for the largest share of the Nitinol Based Medical Device Market, representing nearly 38% of the global market in 2024. The region leads due to strong adoption of advanced medical technologies, high prevalence of cardiovascular and orthopedic disorders, and the presence of key global manufacturers. The United States remains the core market, supported by well-established healthcare infrastructure, early regulatory approvals, and high patient awareness. Hospitals and specialty clinics in the region adopt Nitinol-based stents, catheters, and orthopedic implants on a wide scale, driving consistent growth. Canada contributes to market expansion through growing investments in minimally invasive surgeries and favorable reimbursement policies. It maintains leadership by fostering innovation, supported by collaborations between academic institutions, medical device companies, and healthcare providers.
Europe
Europe holds the second-largest share of the Nitinol Based Medical Device Market, with nearly 28% of the global market in 2024. The region demonstrates strong demand for cardiovascular and neurovascular implants, supported by aging populations and rising cases of chronic conditions. Germany, France, and the United Kingdom serve as the primary contributors due to advanced hospital systems and high adoption of new technologies. The European Union’s regulatory frameworks create challenges in product approvals, but they also drive higher safety standards that build trust among healthcare providers. Increasing investments in research and innovation centers support the development of Nitinol-based orthopedic and dental implants. It remains a robust market for high-quality devices, with steady growth fueled by digital integration in surgical practices and favorable public health initiatives.
Asia Pacific
Asia Pacific secures nearly 22% of the global Nitinol Based Medical Device Market in 2024, positioning it as the fastest-growing regional market. Countries such as China, Japan, and India drive expansion through rising healthcare spending, rapid infrastructure development, and increasing awareness of minimally invasive procedures. Japan leads in early adoption due to its advanced medical research capabilities and strong demand for cardiovascular and orthopedic implants. China emerges as a major growth hub with expanding domestic production capabilities and a large patient population. India demonstrates growing acceptance of Nitinol-based stents and implants, driven by government initiatives to strengthen healthcare delivery. The region attracts global manufacturers seeking to expand their presence in cost-sensitive yet high-potential markets. It strengthens growth momentum through favorable demographics, rising chronic disease burden, and continuous healthcare modernization.
Latin America
Latin America represents about 7% of the global Nitinol Based Medical Device Market in 2024. Brazil and Mexico dominate regional demand, supported by improvements in healthcare infrastructure and growing acceptance of minimally invasive procedures. Rising cardiovascular disease prevalence drives consistent need for Nitinol stents and vascular closure devices. Economic disparities and limited access in some countries restrict widespread adoption, yet ongoing reforms and private investments improve outlook. Local distributors play a vital role in making advanced devices accessible to hospitals and clinics. It continues to grow at a steady pace, driven by urbanization and strengthening healthcare awareness across emerging economies.
Middle East and Africa (MEA)
The Middle East and Africa account for nearly 5% of the global Nitinol Based Medical Device Market in 2024. The region lags behind developed markets but shows gradual adoption supported by healthcare investments in Gulf Cooperation Council (GCC) countries such as Saudi Arabia and the United Arab Emirates. South Africa demonstrates steady demand growth with increasing focus on advanced surgical solutions. Limited infrastructure and high device costs restrict large-scale penetration in lower-income areas, yet public-private partnerships and international collaborations create opportunities. Medical tourism in Middle Eastern nations further boosts the demand for advanced cardiovascular and orthopedic devices. It remains an emerging market with gradual growth, offering long-term potential as healthcare investments accelerate.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Biotronik
- Endosmart
- Boston Scientific
- Arthrex
- Cook Medical
- Becton, Dickinson and Company
- Abbott Laboratories
- Acandis
- B Braun
- Admedes Schuessler
Competitive Analysis
The competitive landscape of the Nitinol Based Medical Device Market is shaped by leading players including Abbott Laboratories, Boston Scientific, Biotronik, B. Braun, Cook Medical, Arthrex, Acandis, Endosmart, Admedes Schuessler, and Becton, Dickinson and Company. These companies drive innovation through extensive product portfolios covering stents, guidewires, catheters, and orthopedic implants. They invest heavily in research and development to enhance device durability, biocompatibility, and performance under complex surgical conditions. Strategic collaborations with hospitals, research institutions, and technology providers support faster product adoption and regulatory clearances. Expansions into emerging markets, especially in Asia Pacific and Latin America, allow them to tap into rising demand from growing patient populations. Competitive strategies also include mergers, acquisitions, and product launches aimed at strengthening global presence and addressing unmet clinical needs. By advancing sustainable solutions and integrating digital technologies into device design, these companies maintain a strong position in a market that demands precision, innovation, and reliability.
Recent Developments
- In August 2025, Abbott Laboratories announced plans to build a new cardiovascular device manufacturing facility in Georgia, USA, expected to complete by 2028, as part of its broader U.S. manufacturing and R&D investment.
- In August 2025, Arthrex received top honors at the Edison Awards for its TightRope SB suture‑based Nitinol implant, designed for ACL reconstruction and expected to launch in Fall 2025
- In February 2025, Biotronik formed a strategic alliance with Egg Medical to co-sell the EggNest™ radiation protection systems in the U.S.
- In January 2025, Biotronik enrolled its first patient in the second arm of the BIO‑CONDUCT IDE trial using the next-generation Solia CSP S pacing lead in the left bundle branch area.
Report Coverage
The research report offers an in-depth analysis based on Product, Application, End Use and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- Major manufacturers invest in next-gen Nitinol alloys to improve fatigue resistance and biocompatibility.
- Personalized implant design grows thanks to 3D modeling and patient-specific manufacturing approaches.
- Growing collaboration between device makers and robotics firms accelerates precision surgery tools.
- Surgeons adopt Nitinol-guided systems paired with real-time imaging to enhance procedural accuracy.
- Developers explore Nitinol-based vascular closure tools for minimally invasive interventions.
- New surface treatments aim to reduce nickel release and improve long-term compatibility.
- Expansion of healthcare infrastructure in emerging markets fosters broader adoption of Nitinol devices.
- Regulatory agencies streamline approval pathways for innovative materials and medical devices.
- Manufacturers integrate smart sensors with Nitinol devices for real-time feedback and remote monitoring.
- Multidisciplinary research supports combining Nitinol with drug delivery technologies for dual-function implants.




