Market Overview:
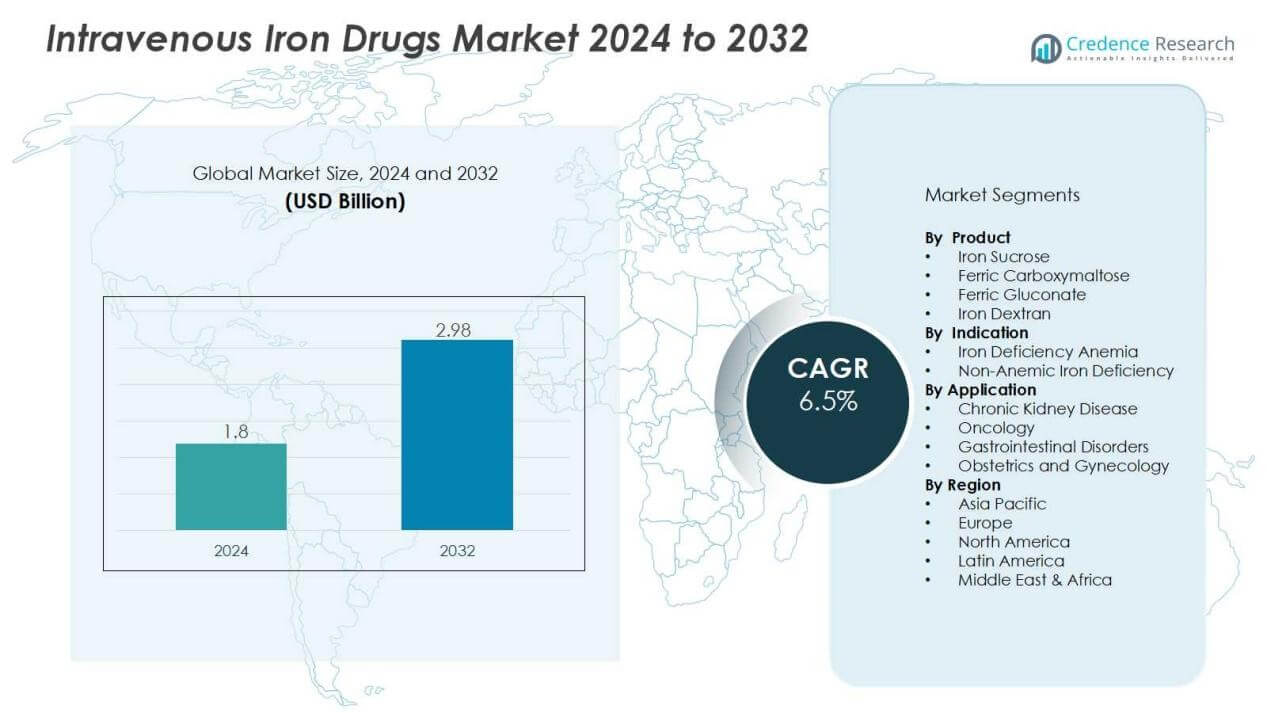
The intravenous iron drugs market size was valued at USD 1.8 billion in 2024 and is anticipated to reach USD 2.98 billion by 2032, at a CAGR of 6.5 % during the forecast period (2024-2032).
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Intravenous Iron Drugs Market Size 2024 |
USD 1.8 Billion |
| Intravenous Iron Drugs Market, CAGR |
6.5% |
| Intravenous Iron Drugs Market Size 2032 |
USD 2.98 Billion |
Key drivers include the growing prevalence of iron deficiency anemia across both developed and developing economies, especially among patients with chronic kidney disease, cancer, and gastrointestinal disorders. Advances in formulations that improve safety and reduce adverse reactions are encouraging wider usage. Expanding awareness among healthcare providers about the clinical benefits of intravenous iron therapy also strengthens market penetration.
Regionally, North America leads the market due to strong healthcare infrastructure, high prevalence of anemia-related conditions, and favorable reimbursement policies. Europe follows closely with significant demand from aging populations and increased adoption of advanced iron therapies. Asia-Pacific is projected to witness the fastest growth, supported by a large patient pool, rising healthcare expenditure, and greater access to hospital-based treatments in countries such as China and India. This regional momentum highlights a global shift toward more effective anemia management solutions.
 Market Insights:
Market Insights:
- The intravenous iron drugs market was valued at USD 1.8 billion in 2024 and is projected to reach USD 2.98 billion by 2032, growing at a CAGR of 6.5% during 2024–2032.
- Rising prevalence of iron deficiency anemia, particularly among patients with chronic kidney disease, cancer, and gastrointestinal disorders, drives strong demand for intravenous formulations.
- Advancements in drug formulations enhance safety, reduce adverse reactions, and improve infusion efficiency, increasing adoption in clinical settings.
- North America held 38% share in 2024, supported by advanced healthcare infrastructure, high anemia prevalence, and favorable reimbursement policies.
- Europe captured 32% share in 2024, driven by strong hospital networks, aging populations, and widespread adoption of innovative therapies.
- Asia-Pacific accounted for 22% share in 2024 and is projected to grow fastest due to rising healthcare spending, diagnostic improvements, and expanding hospital infrastructure.
- High treatment costs and inconsistent reimbursement policies remain key challenges, particularly in emerging markets where affordability restricts patient access.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers:
Rising Prevalence of Iron Deficiency Anemia Across Populations:
The intravenous iron drugs market benefits strongly from the increasing global prevalence of iron deficiency anemia. Millions of patients suffer from this condition due to poor nutrition, blood loss, or chronic illness. Oral iron often fails to provide sufficient absorption, creating a growing demand for intravenous formulations. Healthcare providers recommend these drugs for faster recovery and improved hemoglobin levels. The trend reinforces consistent demand across hospitals and specialty clinics.
- For Instance, A real-world study at the Alrijne Healthcare Group in the Netherlands found that treatment with intravenous ferric derisomaltose (manufactured by Pharmacosmos) increased mean hemoglobin by 14.1 g/L at 4 months in 1,939 adult patients between 2014 and 2021.
Expanding Use in Chronic Kidney Disease and Cancer Patients:
Chronic kidney disease and cancer treatments contribute significantly to the adoption of intravenous iron drugs. These patient groups often experience severe anemia due to impaired iron absorption or blood loss during therapy. It offers rapid and effective restoration of iron stores, reducing the need for repeated blood transfusions. Growing awareness among nephrologists and oncologists supports wider usage. This shift ensures long-term opportunities for manufacturers.
- For Instance, In January 2020, Pharmacosmos’ Monoferric® was approved for single-dose IV iron replacement with a 1000 mg infusion delivered over at least 20 minutes for CKD patients, streamlining anemia management in clinical practice.
Advancements in Formulations and Safety Profiles:
Technological progress in formulations strengthens the appeal of intravenous iron therapies. Modern drugs reduce risks of allergic reactions and minimize side effects compared to earlier versions. It enhances patient compliance and encourages physicians to prefer intravenous options over oral supplements. Faster infusion rates and improved tolerability also increase adoption. Such innovations position these drugs as a safer and more effective alternative.
Growing Healthcare Awareness and Favorable Reimbursement Policies:
Rising healthcare awareness and supportive policies drive market growth in both developed and emerging regions. Governments and insurers in key markets provide reimbursement for intravenous iron treatments, expanding patient access. It encourages hospitals to integrate these drugs into standard care protocols. Education campaigns about anemia management further support demand. These combined efforts strengthen the adoption of intravenous iron drugs across diverse populations.
Market Trends:
Increasing Shift Toward Next-Generation Formulations and Patient-Centric Therapies :
The intravenous iron drugs market is witnessing a clear trend toward next-generation formulations designed to improve safety, efficacy, and infusion convenience. Pharmaceutical companies focus on developing products with lower risks of hypersensitivity reactions and faster infusion times. It enables healthcare providers to treat more patients efficiently while ensuring better compliance. Patient-centric approaches such as single-dose regimens are gaining traction among hospitals and clinics. Rising investments in clinical research support these advancements and strengthen regulatory approvals. Growing physician confidence in modern formulations accelerates their adoption across multiple therapeutic areas.
- For instance, Daiichi Sankyo’s Injectafer (ferric carboxymaltose injection) was approved after clinical trials involving over 8,800 patients globally, with randomized controlled studies of more than 3,500 patients supporting its safety profile—patients were able to receive a single dose up to 750 mg, administered in under 15 minutes.
Expansion in Emerging Economies and Integration with Value-Based Healthcare Models:
Another key trend shaping the market is the rapid expansion of intravenous iron therapies in emerging economies supported by rising healthcare spending and improved access to hospitals. It aligns with the growing emphasis on value-based healthcare models that prioritize cost-effective and efficient anemia management. Governments and private insurers are increasingly recognizing the economic benefits of reducing hospital stays through effective intravenous therapies. Digital health platforms and telemedicine also support patient education and follow-up care, strengthening treatment adoption. Partnerships between global pharmaceutical firms and local healthcare providers accelerate distribution and availability. This broader integration highlights the shift toward accessible and outcome-focused anemia treatment worldwide.
- For instance, in June 2022 Emcure Pharmaceuticals administered ferric carboxymaltose infusions (500–1,000 mg per dose) across 269 centers in India, achieving an average hemoglobin increase of 2.76 g/dL within four weeks of treatment.
Market Challenges Analysis:
Safety Concerns and Adverse Reactions Limiting Wider Adoption:
The intravenous iron drugs market faces significant challenges due to safety concerns and potential adverse reactions. Hypersensitivity, hypotension, and rare anaphylactic responses remain key risks despite advancements in formulations. It discourages some physicians from prescribing intravenous therapies, especially for patients with complex medical conditions. Hospitals must follow strict monitoring protocols during administration, which increases treatment costs and resource requirements. Limited awareness about newer, safer formulations further slows adoption. Addressing these safety barriers is critical for improving physician and patient confidence.
High Costs and Reimbursement Variability Restricting Accessibility:
The high cost of intravenous iron drugs creates affordability issues across several regions. Reimbursement policies remain inconsistent, with partial coverage or limited support in many emerging markets. It limits patient access, particularly in low- and middle-income countries where oral supplements remain the default option. Training requirements for healthcare staff and infrastructure needs also raise overall treatment expenses. Price sensitivity among healthcare providers adds to the challenge, as they often seek lower-cost alternatives. These financial constraints continue to restrict broader market penetration despite clinical advantages.
Market Opportunities:
Expanding Patient Pool and Rising Demand for Effective Anemia Management:
The intravenous iron drugs market holds strong opportunities due to the growing global burden of anemia across diverse populations. Rising prevalence in women of reproductive age, patients with chronic kidney disease, and cancer patients creates sustained demand. It offers a faster and more effective solution compared to oral supplements, making it increasingly favored in clinical settings. Expansion of screening programs for anemia in developing countries also contributes to earlier diagnosis and treatment. Hospitals and clinics are broadening adoption as awareness of patient benefits improves. This expanding patient base ensures long-term growth potential for manufacturers.
Innovation in Formulations and Growing Penetration in Emerging Markets:
Product innovation focused on safer and faster infusions presents a key growth avenue. Pharmaceutical companies are investing in advanced formulations that reduce side effects and enhance patient compliance. It aligns with the rising emphasis on patient-centric healthcare solutions. Emerging markets, particularly in Asia-Pacific, present significant opportunities due to improving healthcare infrastructure and rising disposable incomes. Strategic partnerships with local healthcare providers can accelerate product distribution and adoption. Companies that address both innovation and affordability will gain a competitive edge in capturing these untapped markets.
Market Segmentation Analysis:
By Product
The intravenous iron drugs market is segmented into iron sucrose, ferric carboxymaltose, ferric gluconate, and iron dextran. Ferric carboxymaltose holds a leading position due to its higher safety profile and faster infusion capability. It is preferred in hospital settings for treating large patient volumes efficiently. Iron sucrose remains widely used for chronic kidney disease patients, particularly in dialysis centers. Ferric gluconate continues to gain traction where lower cost options are prioritized. The growing adoption of newer formulations strengthens the product landscape.
- For instance, Ferric carboxymaltose (marketed as Injectafer by American Regent) enables administration of up to 1,500 mg of iron in two infusions of 750 mg each, completed in just 15 minutes per session, which significantly reduces chair time in busy infusion centers.
By Application
Key applications include chronic kidney disease, oncology, gastrointestinal disorders, and obstetrics/gynecology. The chronic kidney disease segment dominates due to the high prevalence of anemia linked to renal dysfunction. It benefits from strong physician preference for intravenous formulations that reduce blood transfusion requirements. Oncology applications are expanding as cancer patients often face treatment-induced anemia. Gastrointestinal disorders drive demand where oral iron absorption is poor. Rising focus on women’s health supports uptake in obstetrics and gynecology care.
- For instance, Amgen’s EPOGEN (epoetin alfa) is approved for anemia in chronic kidney disease (CKD) patients on dialysis to reduce their need for red blood cell transfusions. An estimated 778,000 U.S. patients received treatment with Epogen between its launch in June 1989 and December 2020.
By Indication
Segmentation by indication covers iron deficiency anemia and non-anemic iron deficiency. Iron deficiency anemia represents the largest segment due to its global prevalence across varied patient groups. It drives consistent use of intravenous therapies when oral supplements prove ineffective. Non-anemic iron deficiency, though smaller, is growing with rising awareness and diagnostic improvements. Healthcare providers increasingly recognize its role in improving patient outcomes. This segment’s expansion highlights opportunities for broader treatment adoption.
Segmentations:
By Product:
- Iron Sucrose
- Ferric Carboxymaltose
- Ferric Gluconate
- Iron Dextran
By Application:
- Chronic Kidney Disease
- Oncology
- Gastrointestinal Disorders
- Obstetrics and Gynecology
By Indication:
- Iron Deficiency Anemia
- Non-Anemic Iron Deficiency
By Region:
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis:
North America:
North America accounted for 38% market share in 2024, making it the largest regional contributor. The intravenous iron drugs market in this region benefits from advanced healthcare infrastructure and robust insurance coverage. It is driven by high prevalence of chronic kidney disease and anemia among aging populations. Favorable reimbursement policies encourage hospitals and clinics to integrate intravenous iron into treatment protocols. Pharmaceutical companies focus heavily on the U.S. and Canada due to their high treatment adoption rates. Continuous product launches and physician awareness programs reinforce the region’s dominance.
Europe:
Europe held 32% market share in 2024, securing its position as the second-largest market globally. The intravenous iron drugs market benefits from stringent treatment guidelines and widespread availability across public healthcare systems. It is supported by rising cases of anemia linked to aging populations and chronic illnesses. Countries such as Germany, France, and the UK lead adoption due to strong hospital networks. Expansion of innovative drug formulations with better safety profiles accelerates usage. Ongoing research collaborations and favorable regulatory approvals strengthen the market outlook in this region.
Asia-Pacific:
Asia-Pacific recorded 22% market share in 2024 and represents the fastest-growing region. The intravenous iron drugs market gains momentum from large patient pools in China, India, and Southeast Asia. It is driven by rising healthcare investments, improved diagnostic rates, and expanding hospital infrastructure. Growing awareness about effective anemia management fuels demand for advanced therapies. Pharmaceutical companies expand through partnerships with local distributors to widen access. Rising disposable incomes and urbanization further enhance treatment adoption, positioning Asia-Pacific as a key growth engine for the future.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- AbbVie Inc.
- AMAG Pharmaceuticals (Covis Pharma)
- Daiichi Sankyo Company, Ltd.
- Sanofi
- Vifor Pharma Management Ltd. (CSL)
- PHARMACOSMOS A/S
- Zydus Group
- Rockwell Medical, Inc.
- Allergan Plc.
Competitive Analysis:
The intravenous iron drugs market is defined by strong competition among global and regional pharmaceutical companies. Key players include AbbVie Inc., AMAG Pharmaceuticals (Covis Pharma), Daiichi Sankyo Company, Ltd., Sanofi, and Vifor Pharma Management Ltd. (CSL). These companies focus on expanding portfolios with advanced formulations that improve safety and infusion speed. It drives adoption in hospitals and specialty clinics where patient demand for efficient anemia treatment continues to rise. Strategic alliances, regulatory approvals, and product innovation remain central to sustaining competitive advantage. Market leaders invest in clinical research to strengthen physician confidence and differentiate their products. Expansion into emerging markets through distribution agreements and local partnerships enhances growth opportunities. Competition is also shaped by pricing strategies and reimbursement policies, which influence accessibility across regions. This competitive environment highlights innovation, affordability, and global reach as the primary factors defining long-term success.
Recent Developments:
- In August 2025, Daiichi Sankyo and Merck announced that their antibody-drug conjugate Ifinatamab Deruxtecan received FDA Breakthrough Therapy designation for small cell lung cancer, reinforcing their ongoing collaboration in oncology innovation.
- In July 2025, Sanofi completed the acquisition of Blueprint Medicines Corporation, adding Ayvakit/Ayvakyt and an immunology pipeline to its portfolio.
Report Coverage:
The research report offers an in-depth analysis based on Product, Application, Indication and Region. It details leading Market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current Market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven Market expansion in recent years. The report also explores Market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on Market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the Market.
Future Outlook:
- The intravenous iron drugs market will expand steadily, driven by rising anemia prevalence worldwide.
- It will see increasing adoption in chronic kidney disease and cancer patients requiring efficient anemia management.
- Innovation in formulations will focus on improving safety, reducing side effects, and enhancing infusion speed.
- Healthcare providers will continue shifting from oral supplements to intravenous options for improved clinical outcomes.
- North America and Europe will maintain leadership due to advanced healthcare systems and supportive reimbursement.
- Asia-Pacific will emerge as the fastest-growing region with expanding hospital infrastructure and diagnostic access.
- Collaborations between pharmaceutical firms and local distributors will strengthen global availability and distribution networks.
- Digital health platforms will support patient education, adherence monitoring, and follow-up care for better outcomes.
- Regulatory approvals for new formulations will accelerate product adoption and boost physician confidence in therapies.
- Sustainability in pricing and broader affordability initiatives will shape future accessibility in emerging economies.

 Market Insights:
Market Insights:

