Market Overview:
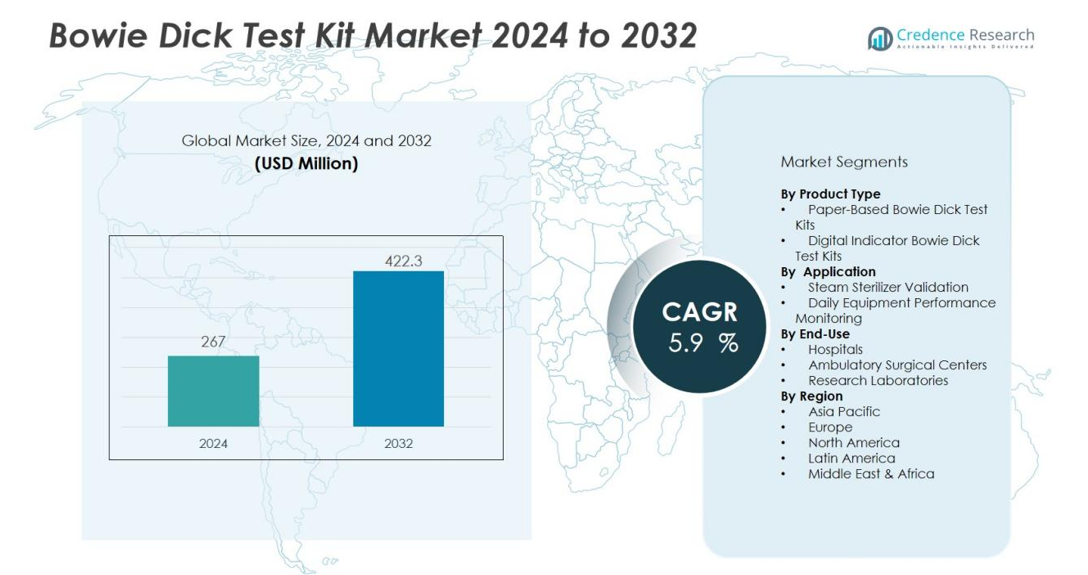
The Bowie-Dick Test Kit Market size was valued at USD 267 million in 2024 and is anticipated to reach USD 422.3 million by 2032, at a CAGR of 5.9 % during the forecast period (2024-2032).
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Bowie-Dick Test Kit Market Size 2024 |
USD 267 million |
| Bowie-Dick Test Kit Market, CAGR |
5.9% |
| Bowie-Dick Test Kit Market Size 2032 |
USD 422.3 million |
Key market drivers include the growing prevalence of healthcare-associated infections, which necessitate strict sterilization standards across hospitals, clinics, and laboratories. Advancements in test kit design, offering rapid and accurate results, improve workflow efficiency and support compliance with international sterilization standards such as ISO 11140-4. The expansion of hospital infrastructure and the increasing number of surgical procedures further boost demand for reliable sterilization monitoring solutions.
Regionally, North America dominates the Bowie Dick test kit market, supported by rigorous regulatory requirements and a large network of accredited healthcare institutions. Europe follows, propelled by established healthcare systems and high emphasis on infection control. Asia-Pacific demonstrates the fastest growth due to expanding healthcare investments, increasing awareness of infection prevention, and rapid modernization of medical facilities across emerging economies.
Market Insights:
- The bowie dick test kit market reached USD 267 million in 2024 and is forecasted to hit USD 422.3 million by 2032.
- Strict sterilization standards and regulatory requirements drive steady market demand across hospitals, clinics, and laboratories.
- Rising healthcare-associated infections push adoption of effective sterilization monitoring tools to ensure patient safety.
- Technological advancements in test kit design deliver rapid, accurate results and support digital compliance reporting.
- North America leads with 34% market share, benefiting from advanced healthcare systems and high regulatory oversight.
- Europe secures 28% share, with strong infection control protocols and emphasis on eco-friendly product development.
- Asia-Pacific holds 24% share and records the fastest growth, propelled by healthcare infrastructure expansion and increased awareness of infection prevention.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers:
Strict Sterilization Standards and Regulatory Compliance:
Stringent sterilization standards drive the bowie dick test kit market across healthcare facilities worldwide. Hospitals and clinics must comply with national and international regulations to ensure patient safety and infection control. Regulatory bodies such as the CDC and ISO require regular steam penetration testing for autoclaves. The market benefits from mandatory adoption of Bowie Dick test kits as part of routine quality assurance protocols in sterilization departments.
- The CDC specifically mandates that the Bowie-Dick test be performed daily for pre-vacuum sterilizers, running a test pack at 134°C for 3.5 minutes to ensure effective air removal and steam penetration.
Rising Incidence of Healthcare-Associated Infections:
The increasing prevalence of healthcare-associated infections (HAIs) strengthens demand for effective sterilization validation tools. The bowie dick test kit market responds to growing pressure on healthcare providers to minimize infection risks during medical procedures. It supports consistent performance checks for sterilization equipment, helping organizations achieve high standards of hygiene. Hospitals and surgical centers prioritize these kits to prevent cross-contamination and protect patient health.
- For instance, according to the Centers for Disease Control and Prevention, approximately 1 in every 31 hospitalized patients in the U.S.—about 687,000 people annually—contract an HAI, driving hospitals to intensify sterilization processes.
Technological Advancements in Test Kit Design:
Innovations in test kit materials and indicator technologies enhance the reliability and speed of Bowie Dick test results. The bowie dick test kit market benefits from user-friendly formats that reduce operator errors and deliver clear results. Improvements in sensitivity and specificity enable early detection of sterilization failures. It supports integration with digital recordkeeping systems, making compliance and audits more efficient for healthcare institutions.
Expansion of Healthcare Infrastructure in Emerging Markets:
Expanding healthcare infrastructure in developing regions fuels the bowie dick test kit market’s growth. New hospital construction and upgrades in sterilization departments create strong demand for steam penetration monitoring solutions. Governments and private investors invest in modern medical facilities equipped with advanced infection control technologies. The market capitalizes on rising awareness and adoption of international sterilization practices in Asia-Pacific, Latin America, and the Middle East.
Market Trends:
Integration of Digital Monitoring and Smart Technologies:
The bowie dick test kit market sees increasing adoption of digital monitoring solutions and smart test kits. Manufacturers introduce kits equipped with advanced sensors and connectivity features that enable automated tracking and data logging of sterilization cycles. Healthcare facilities seek solutions that allow real-time result capture and integration with hospital management systems for streamlined compliance reporting. Automated digital indicators reduce the risk of human error and support remote quality audits. It drives operational efficiency for central sterile service departments by minimizing manual documentation tasks. Growing demand for traceability and transparency in sterilization processes accelerates the shift toward digital Bowie Dick test kits across leading hospitals and laboratories.
- For Instance, data logger solutions such as the TrackSense Pro XL by Ellab introduce real-time wireless data transmission. This technology can constantly monitor sterilization processes at both 121°C and 134°C, enabling rapid feedback on critical sterilization parameters—temperature, humidity, vacuum, and steam penetration—and provides electronic audit trails for instant validation and compliance.
Emphasis on Sustainability and Eco-Friendly Product Development:
Sustainability remains a strong trend in the bowie dick test kit market, with manufacturers focusing on environmentally responsible materials and processes. Companies develop biodegradable and recyclable test sheets to minimize the environmental footprint of single-use products. Hospitals and clinics prefer test kits that meet both regulatory and green procurement standards. The market benefits from growing preference for non-toxic indicator chemicals and low-waste packaging. Innovation centers on reducing hazardous waste generation without compromising test reliability or accuracy. It positions eco-friendly Bowie Dick test kits as a preferred choice for healthcare providers aiming to meet sustainability goals while ensuring rigorous sterilization quality control.
Market Challenges Analysis:
Cost Constraints and Budgetary Limitations in Healthcare Settings:
The bowie dick test kit market faces challenges from cost constraints and tight budgets across hospitals and clinics. Many healthcare facilities, especially in emerging economies, must balance quality assurance spending with other operational priorities. High-quality Bowie Dick test kits can increase recurring expenses for sterilization departments. Procurement teams often evaluate less expensive alternatives, which may compromise reliability or regulatory compliance. Public hospitals and smaller clinics face the greatest difficulties in justifying the adoption of premium test kits. It creates barriers to widespread implementation, particularly in regions with limited healthcare funding.
Limited Awareness and Training on Proper Use:
A significant challenge for the bowie dick test kit market remains limited awareness and training among healthcare workers. Proper use and interpretation of Bowie Dick test results require specialized knowledge and routine practice. Inconsistent training can lead to errors in test execution or misinterpretation of results, risking undetected sterilization failures. Variability in adherence to standard protocols across facilities impacts overall effectiveness. The market must address the need for comprehensive user education to ensure optimal outcomes. It underscores the importance of ongoing training and clear instructions from manufacturers.
Market Opportunities:
Growth Potential in Emerging Healthcare Markets:
The bowie dick test kit market presents strong opportunities in rapidly expanding healthcare systems across Asia-Pacific, Latin America, and Africa. Governments and private investors invest in new hospitals, clinics, and laboratories, creating demand for reliable sterilization monitoring solutions. The shift toward international accreditation and compliance with global infection control standards supports market growth. Local distributors and global manufacturers can form strategic partnerships to penetrate underserved regions. It enables increased access to advanced sterilization technologies for healthcare providers in developing markets. Rising healthcare expenditure and public health initiatives further boost adoption.
Expansion into Digital and Smart Product Segments:
The bowie dick test kit market offers new opportunities through the introduction of digital and smart test kits. Demand grows for products that support automated result recording, remote monitoring, and integration with electronic health record systems. Manufacturers investing in digital transformation position themselves as leaders in next-generation sterilization assurance. It appeals to healthcare institutions focused on efficiency, accuracy, and regulatory compliance. The market can capitalize on trends in digital health and the need for traceability in quality assurance processes. Rapid advancements in connected technologies accelerate product innovation and create competitive differentiation.
Market Segmentation Analysis:
By Product Type
The bowie dick test kit market divides into conventional paper-based kits and advanced digital indicator kits. Conventional kits hold the largest share due to widespread use and cost-effectiveness in routine sterilization checks. Digital indicator kits are gaining traction as healthcare facilities shift toward automated monitoring and data integration. It benefits from the reliability and ease of use that both types offer, allowing facilities to select products that match their workflow requirements and compliance needs.
- For instance, the Getinge Check Bowie-Dick Mini Pack 134°C is a widely used paper-based test that features a clear color change from purple to green and is designed for ease of use, conforming to EN ISO 11140-4 standards. Hospitals utilize such packs daily to verify air removal in prevacuum sterilizers, typically processing at 134°C.
By Application
The bowie dick test kit market segments by application into steam sterilizer validation and daily equipment performance monitoring. Steam sterilizer validation remains the dominant application, driven by regulatory mandates for daily verification of autoclave efficiency in hospitals and laboratories. Daily performance monitoring supports quality assurance protocols and helps prevent equipment failures. It addresses the need for consistent sterilization outcomes, supporting patient safety and regulatory compliance.
- For instance, Ebro’s EBI-100 data loggers, widely deployed in U.S. healthcare facilities, provide high-precision measurements with temperature accuracy up to 0.05°C and are capable of real-time monitoring in steam sterilizers to ensure each cycle consistently meets sterilization standards.
By End-Use
The bowie dick test kit market serves hospitals, ambulatory surgical centers, and research laboratories. Hospitals account for the largest end-use segment, fueled by high patient volumes and strict infection control standards. Ambulatory surgical centers adopt these kits to maintain accreditation and ensure effective sterilization for outpatient procedures. Research laboratories also require frequent sterilization checks to protect sample integrity and maintain safe work environments. It supports a diverse user base focused on quality and safety in sterilization processes.
Segmentations:
By Product Type:
- Paper-Based Bowie Dick Test Kits
- Digital Indicator Bowie Dick Test Kits
By Application:
- Steam Sterilizer Validation
- Daily Equipment Performance Monitoring
By End-Use:
- Hospitals
- Ambulatory Surgical Centers
- Research Laboratories
By Region:
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
North America:
North America holds 34% market share in the bowie dick test kit market, driven by advanced healthcare infrastructure and strict regulatory oversight. The region benefits from a large network of accredited hospitals and centralized sterile processing departments. High awareness of infection prevention and established sterilization protocols secure consistent demand for test kits. Major manufacturers operate local production facilities and distribution channels, ensuring reliable product availability. The United States leads market activity, with Canada contributing a significant portion through public healthcare investments. It maintains high compliance rates with CDC and ISO sterilization guidelines, sustaining long-term adoption.
Europe:
Europe commands 28% market share in the bowie dick test kit market, supported by comprehensive healthcare regulations and widespread infection control initiatives. Countries such as Germany, France, and the United Kingdom implement strict sterilization standards across healthcare settings. Investments in public health infrastructure and routine audits drive steady demand for Bowie Dick test kits. Leading European manufacturers emphasize eco-friendly product development to align with regional sustainability goals. The market benefits from strong distributor networks and government-backed awareness programs. It responds well to new technological advancements and digital integration in sterilization monitoring.
Asia-Pacific:
Asia-Pacific holds 24% market share in the bowie dick test kit market and records the highest growth rate among all regions. Rapid expansion of healthcare infrastructure and increasing numbers of surgical procedures fuel demand for effective sterilization monitoring tools. China, India, and Japan serve as major contributors due to their large populations and rising hospital construction projects. Growing emphasis on international accreditation and infection control compliance stimulates market penetration. Global and regional players invest in local manufacturing and education initiatives to support adoption. It benefits from rising healthcare expenditures and government-backed modernization programs across emerging economies.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- Medline Industries Inc.
- Crosstie International Inc.
- EFELAB SRL
- 4Crosstex Air View
- Getinge AB
- Steris
- EDM3 Health Link
- PMS Medical
- Clinic hem
- Propper Manufacturing Co. Inc.
Competitive Analysis:
The bowie dick test kit market features strong competition among established players and emerging specialists. Key companies include Medline Industries Inc., Crosstie International Inc., EFELAB SRL, 4Crosstex Air View, Getinge AB, and Steris, each offering solutions tailored to rigorous sterilization standards. It rewards suppliers that deliver consistent product quality, regulatory compliance, and robust customer support. Leading firms invest in digital innovation and sustainable product development to differentiate their portfolios. Competitive strategies focus on expanding distribution networks, improving operator training, and introducing user-friendly, technologically advanced kits. It encourages continuous improvement and responsiveness to evolving healthcare regulations and facility requirements. Strong brand reputation and global presence help market leaders capture long-term contracts with hospitals and healthcare groups, sustaining their market positions.
Recent Developments:
- In July 2025, Medline Industries Inc. launched the ComfortTemp® Patient Warming System, which includes blankets and gowns to enhance patient comfort and outcomes.
- In August 2024, The pharmaceutical industry, including partnerships involving PMS Medical, emphasized the ongoing collaboration between Product Managers and Medical Science Liaisons as a best practice in product development and strategy guidance.
- In September 2024, Propper Manufacturing Co. Inc. announced the launch of EO Chex™ Indicator Tape, becoming the only FDA-cleared ethylene oxide indicator tape available for sale in the United States.
Market Concentration & Characteristics:
The bowie dick test kit market features moderate concentration, with several global and regional manufacturers competing for share. It includes a mix of established players and specialized suppliers, each offering products that meet strict international standards for sterilization monitoring. Companies focus on product innovation, reliability, and compliance with regulatory guidelines to maintain a competitive edge. The market favors suppliers that provide user-friendly kits and comprehensive technical support to healthcare institutions. It responds quickly to evolving demands for digital integration and sustainable materials. Consistent demand from hospitals and laboratories sustains steady growth and encourages ongoing investment in quality and technological advancement.
Report Coverage:
The research report offers an in-depth analysis based on Product Type, Application, End-Use and Region. It details leading Market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current Market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven Market expansion in recent years. The report also explores Market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on Market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the Market.
Future Outlook:
- Demand from hospitals and surgical centers will rise due to increasing emphasis on sterilization validation and infection control.
- Healthcare facilities will integrate digital Bowie Dick test kits into electronic health record systems for automated data tracking.
- Manufacturers will invest in eco-friendly test materials and recyclable packaging to align with environmental procurement policies.
- Emerging economies will present strong adoption opportunities as new medical facilities launch and upgrade their sterilization departments.
- Suppliers will expand training programs for sterilization staff to ensure proper use and accurate interpretation of test results.
- Strategic partnerships between global brands and regional distributors will improve access in underserved markets.
- Adoption of connected test indicators will grow, enabling remote monitoring, audit readiness, and real-time compliance reporting.
- Innovation in indicator chemistry and design will enhance sensitivity and reduce operator error in sterility assessments.
- Regulatory agencies will tighten sterilization standards, increasing demand for first‑pass validation tools.
- Healthcare providers will prioritize traceability and audit documentation, driving the shift toward digital and smart test kits.




