Market Overview
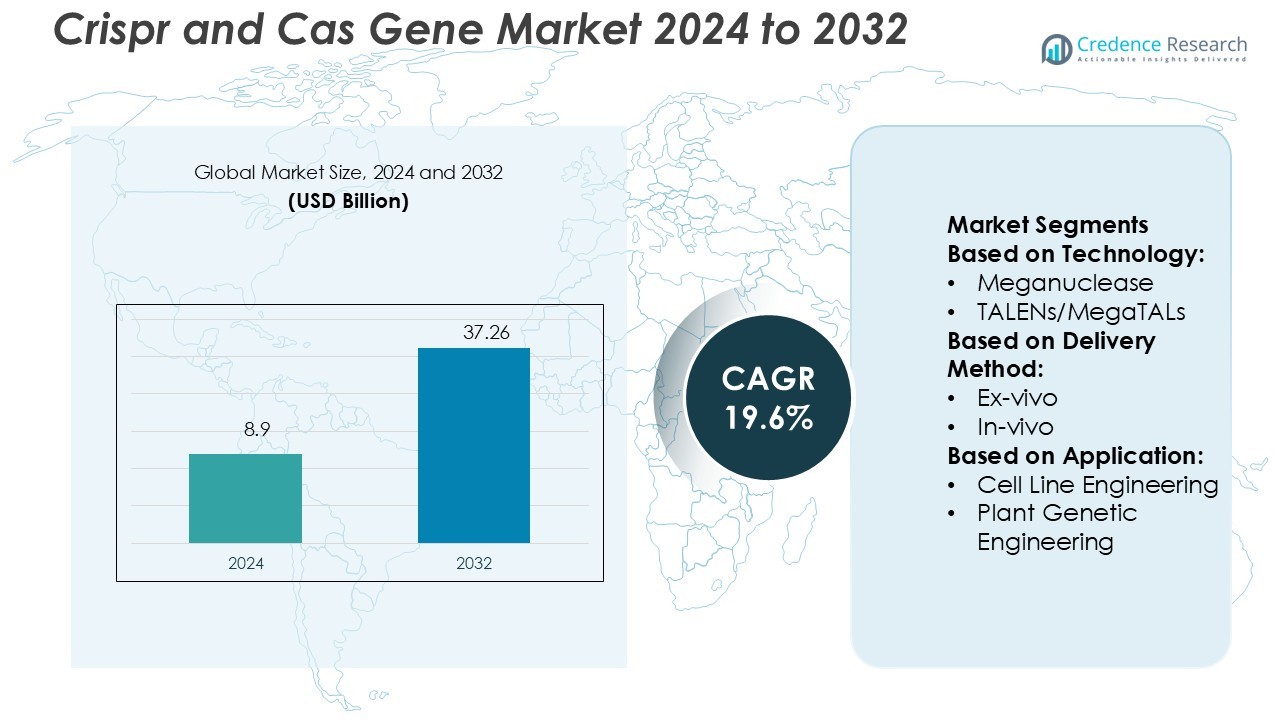
Crispr and Cas Gene Market size was valued USD 8.9 billion in 2024 and is anticipated to reach USD 37.26 billion by 2032, at a CAGR of 19.6% during the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| CRISPR and Cas Gene Market Size 2024 |
USD 8.9 Billion |
| CRISPR and Cas Gene Market , CAGR |
19.6% |
| CRISPR and Cas Gene Market Size 2032 |
USD 37.26 Billion |
The CRISPR and Cas Gene market is led by major players including Synthego, Thermo Fisher Scientific, GenScript, Origene Technologies, Lonza, Illumina, Revvity, Inc., Merck KGaA, Agilent Technologies, and Danaher. These companies focus on expanding their gene-editing capabilities through advanced product development, R&D investments, and strategic partnerships with academic and biotech institutions. They emphasize improving precision, efficiency, and scalability of CRISPR technologies for clinical and agricultural applications. North America holds the leading position in the global market with a 38% share, supported by strong research infrastructure, favorable regulations, and high adoption of genomic therapies. The presence of key industry innovators and rapid clinical trial activities further strengthen the region’s dominance.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights
- The CRISPR and Cas Gene Market was valued at USD 8.9 billion in 2024 and is expected to reach USD 37.26 billion by 2032, growing at a CAGR of 19.6%.
- Rising demand for gene-editing therapies and increasing investment in R&D are driving strong market growth across healthcare and agriculture.
- Advancements in precision technologies and strategic collaborations are creating new opportunities, with major players expanding global reach.
- High development costs and regulatory complexities remain key restraints, impacting smaller biotech firms and slowing product approvals.
- North America leads the market with a 38% share, supported by advanced research infrastructure and rapid clinical adoption, while ex-vivo delivery and CRISPR/Cas9 technology segments dominate global revenue.
Market Segmentation Analysis:
By Technology
The (CRISPR)/Cas9 segment holds the largest share of the CRISPR and Cas Gene Market. This dominance comes from its high editing precision, efficiency, and ease of use compared to ZFN, TALENs, Meganuclease, and others. The technology is widely adopted for gene knockout, transcription regulation, and genome-wide screening. Its adaptability across plants, animals, and human cells strengthens its market leadership. Continuous advancements, such as high-fidelity Cas9 variants and base editing tools, improve accuracy and reduce off-target effects. Major biotechnology firms and academic research programs favor Cas9 for its scalability and flexibility in therapeutic development.
- For instance, Synthego developed engineered sgRNA libraries enabling CRISPR/Cas9 editing in over 200 different human cell lines, with editing efficiencies exceeding 90% in standardized assays.
By Delivery Method
The ex-vivo delivery segment accounts for the largest market share. This method offers better control over gene editing conditions and minimizes unintended mutations. Ex-vivo is widely applied in developing CAR-T cell therapies, stem cell modifications, and immune system reprogramming. Pharmaceutical companies and research institutions use it to develop precise and safer therapies for cancer and genetic disorders. Strong clinical trial pipelines and favorable regulatory approvals further strengthen its adoption. In-vivo delivery, while growing, faces higher safety and delivery complexity compared to ex-vivo approaches.
- For instance, Thermo Fisher Scientific has engineered the CTS Xenon Electroporation System to support ex-vivo editing of up to 2.5 billion T cells in a single 25 mL run in a closed format.
By Application
The therapy development segment leads the CRISPR and Cas Gene Market. This dominance is driven by increasing use in treating genetic diseases, cancer, and rare disorders. Companies are focusing on developing CRISPR-based therapies targeting inherited conditions such as sickle cell anemia and muscular dystrophy. The technology enables targeted modifications in disease-causing genes with high precision. Diagnostics and genetic engineering also hold notable shares, but therapy development attracts the highest investments. The rise in clinical trials and collaborations between biotech firms and research institutes continues to accelerate this segment’s growth.
Key Growth Drivers
Rising Demand for Gene Therapy Solutions
The growing demand for advanced gene therapy drives the CRISPR and Cas Gene Market. CRISPR-based tools enable precise DNA modification, supporting the treatment of genetic disorders such as sickle cell anemia and muscular dystrophy. Pharmaceutical companies are increasing investments to develop targeted therapies with improved safety and efficacy. Expanding clinical trials and early regulatory support strengthen confidence in CRISPR technology. The ability to address previously untreatable conditions positions CRISPR as a core tool in modern healthcare innovation.
- For instance, GenScript’s services boast up to 97 % editing efficiency for Cas9 systems and up to 98 % for Cas12a in cell models. CRISPR sgRNAs of length 97–103 nucleotides with full chemical synthesis.
Expanding Applications Across Research and Agriculture
CRISPR technology is rapidly adopted across biotechnology, agriculture, and industrial research. Researchers use it to improve crop yields, create disease-resistant plants, and engineer animal models. Academic institutions and agri-biotech companies integrate gene editing to accelerate product development. The rising demand for sustainable agriculture and food security supports broader use. Flexible technology design, reduced development time, and lower costs increase its appeal in non-therapeutic fields, driving long-term market expansion.
- For instance, OriGene offers KN2.0 non-homology mediated knockout kits that achieve high biallelic editing in both dividing and nondividing cells. CRISPR knockout kits include 2 gRNA vectors plus donor DNA cassettes to facilitate knockout screening.
Technological Advancements and Strategic Collaborations
Advancements in base editing, prime editing, and delivery systems enhance CRISPR’s precision and safety. Companies and research institutes are forming strategic alliances to expand applications and accelerate commercialization. These collaborations support faster clinical progress and regulatory approval. Investment in next-generation CRISPR platforms strengthens market leadership positions. Enhanced efficiency and reduced off-target effects make the technology more viable for clinical and industrial applications, boosting market confidence and growth.
Key Trends & Opportunities
Growing Pipeline of CRISPR-Based Therapeutics
The CRISPR and Cas Gene Market is witnessing rapid growth in therapy pipelines targeting cancer, rare diseases, and genetic disorders. Numerous biotech companies are advancing CRISPR therapies through late-stage clinical trials. The approval of first-generation therapies encourages further investments. Expanding applications in personalized medicine and precision oncology create significant opportunities. Strong R&D funding from public and private sources accelerates commercialization timelines.
- For instance, Lonza secured a long-term manufacturing agreement with Vertex to produce CASGEVY®, the first approved CRISPR/Cas-9 gene-edited therapy, at its cGMP cell therapy site in Geleen.
Rising Interest in Agriculture and Food Security
CRISPR technology is gaining traction in agriculture for improving crop resilience, yield, and quality. Governments and agri-tech firms are promoting gene-edited crops to meet rising food demand. Fast regulatory pathways in some regions enable quicker market entry. Sustainable farming practices and climate-resilient crops strengthen this opportunity. The trend supports diversification beyond human therapeutics, expanding the total addressable market.
- For instance, Revvity’s gene delivery arm will combine its AAV vector technology with GenKOre’s compact TaRGET platform (1.7 kb nuclease module vs Cas9’s 4.1 kb) to develop in vivo therapies for ocular disorders like LCA10 and USH2A.
Integration of AI and Automation in CRISPR Research
AI-driven design tools and automated platforms are improving CRISPR target prediction and editing precision. Automation accelerates experimental workflows, reducing development costs and timelines. Companies are integrating these tools to enhance accuracy and scalability in gene editing projects. The trend is expected to drive innovation and create a competitive advantage for technology-driven players.
Key Challenges
Regulatory and Ethical Concerns
Regulatory complexity and ethical debates surrounding gene editing pose major challenges. Concerns over human germline editing and unintended consequences lead to stricter oversight. Global regulatory variations create uncertainty for product development and commercialization. Balancing innovation with compliance requires significant investment and time. These factors may delay approvals and limit rapid market growth.
Off-Target Effects and Technical Limitations
Despite rapid progress, off-target mutations remain a key technical hurdle. Inaccurate edits can lead to unwanted genetic changes, raising safety concerns. Developing more precise tools and improving delivery methods require extensive R&D investments. Technical barriers can slow clinical translation and reduce market confidence. Overcoming these limitations is essential to ensure wider acceptance of CRISPR-based solutions.
Regional Analysis
North America
North America leads the CRISPR and Cas Gene market with a 38% market share. Strong funding from government bodies and private investors drives rapid research adoption. The region benefits from advanced biotech infrastructure, favorable regulatory policies, and a large base of gene therapy developers. The U.S. plays a key role, with leading institutes accelerating clinical trials and commercialization. Companies like CRISPR Therapeutics, Editas Medicine, and Intellia Therapeutics are expanding gene-editing pipelines. Rising demand for personalized medicine, along with supportive FDA guidance, continues to boost regional growth and global competitiveness.
Europe
Europe accounts for a 29% share of the CRISPR and Cas Gene market. Strong R&D frameworks and cross-border collaborations support clinical and agricultural applications. Nations such as Germany, France, and the UK lead innovation with well-established gene editing regulations. Public-private partnerships enhance trial approvals and product commercialization. Major biopharmaceutical firms are investing in precision gene therapy and crop engineering. EU regulatory clarity and funding initiatives strengthen the competitive landscape. The region’s focus on ethical gene editing and sustainable agriculture also positions it as a critical global hub.
Asia Pacific
Asia Pacific holds a 24% market share, driven by rising biotechnology investments and expanding genomics research. China, Japan, and South Korea are key contributors with government-backed funding and talent-rich research ecosystems. Rapid clinical trial expansion, coupled with agricultural applications, boosts adoption. Local firms collaborate with global biotech leaders to accelerate gene therapy development. The growing healthcare infrastructure and large patient base support therapeutic testing. Regulatory frameworks are evolving quickly to match innovation speed, further enhancing Asia Pacific’s position in the global CRISPR and Cas Gene landscape.
Latin America
Latin America represents 5% of the global market, with growth centered on agricultural and academic research applications. Brazil, Mexico, and Argentina are investing in CRISPR technologies for crop yield enhancement and disease resistance. Public research institutions are playing a significant role in technology transfer and partnerships. Although regulatory frameworks are still developing, pilot projects in agriculture and rare disease research are increasing. Lower R&D costs and improving biotech infrastructure support gradual adoption. International collaborations are helping the region build capacity and expand participation in global CRISPR projects.
Middle East & Africa
The Middle East & Africa holds a 4% market share in the CRISPR and Cas Gene market. Regional growth is supported by rising investments in precision medicine and genomics. Countries such as the UAE, Saudi Arabia, and South Africa are enhancing research capabilities through partnerships with global institutions. Academic and healthcare institutions focus on genetic disease screening and agricultural biotechnology. Government initiatives aim to modernize healthcare infrastructure and build biotech clusters. While adoption is at an early stage, strong investment momentum indicates steady market expansion in the coming years.
Market Segmentations:
By Technology:
- Meganuclease
- TALENs/MegaTALs
By Delivery Method:
By Application:
- Cell Line Engineering
- Plant Genetic Engineering
By Geography
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Competitive Landscape
The CRISPR and Cas Gene market is shaped by leading players such as Synthego, Thermo Fisher Scientific, GenScript, Origene Technologies, Lonza, Illumina, Revvity, Inc., Merck KGaA, Agilent Technologies, and Danaher. The CRISPR and Cas Gene market is defined by strong innovation, strategic collaborations, and rapid commercialization of advanced gene-editing solutions. Companies are focusing on expanding their research capabilities, improving precision technologies, and developing scalable platforms to meet rising global demand. Many are investing in automation, AI-driven analysis, and high-throughput screening to enhance efficiency and reduce development timelines. Strategic partnerships with academic institutions and biotech firms are accelerating clinical applications across healthcare and agriculture. Firms are also prioritizing regulatory compliance, ethical standards, and secure intellectual property strategies to strengthen their market position and maintain a competitive edge.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Key Player Analysis
Recent Developments
- In July 2025, Profluent launched an AI model that can generate CRISPR proteins. Through this launch, the company claimed that the usage of AI can create a large number of CRISPR proteins that help manufacture bespoke cures.
- In May 2025, Aldevron and Integrated DNA Technologies (IDT) delivered the world’s first personalized mRNA CRISPR therapy for an infant with a urea cycle disorder (UCD) in a record-breaking six months.
- In April 2024, Regeneron Pharmaceuticals collaborated with Mammoth Biosciences. This collaboration is done to use Mammoth’s platform with Regeneron to develop in vivo therapies for tissues.
- In January 2024, Danaher Corporation collaborated with Innovative Genomics Institute (IGI). This collaboration aimed to develop gene editing therapies for the treatment of rare diseases and other diseases.
Report Coverage
The research report offers an in-depth analysis based on Technology, Delivery Method, Application and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- The market will expand with increasing adoption of gene-editing therapies in healthcare.
- Advancements in precision editing will improve treatment outcomes for genetic disorders.
- AI and automation will streamline research, reducing development timelines.
- Strategic collaborations will accelerate clinical trials and regulatory approvals.
- Agricultural applications will grow, supporting sustainable farming and food security.
- Ethical and regulatory frameworks will strengthen, ensuring safe global adoption.
- Cost-effective gene-editing tools will make advanced therapies more accessible.
- Investments in R&D will drive breakthroughs in delivery systems and efficiency.
- Personalized medicine will become a major focus area for market growth.
- Global partnerships will expand access to CRISPR technologies in emerging regions.




