CHAPTER NO. 1 : INTRODUCTION 19
1.1.1. Report Description 19
Purpose of the Report 19
USP & Key Offerings 19
1.1.2. Key Benefits for Stakeholders 19
1.1.3. Target Audience 20
1.1.4. Report Scope 20
CHAPTER NO. 2 : EXECUTIVE SUMMARY 21
2.1. Germany Medical Device Contract Manufacturing Market Snapshot 21
2.1.1. Germany Medical Device Contract Manufacturing Market, 2018 – 2032 (USD Million) 22
CHAPTER NO. 3 : GERMANY MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – INDUSTRY ANALYSIS 23
3.1. Introduction 23
3.2. Market Drivers 24
3.2.1. Strong Regulatory Framework and Compliance Standards 24
3.2.2. Aging Population and Increasing Demand for Healthcare Solutions 25
3.3. Market Restraints 26
3.3.1. Increasing Competition from Low-Cost Manufacturing 26
3.4. Market Opportunities 27
3.4.1. Market Opportunity Analysis 27
3.5. Porter’s Five Forces Analysis 28
CHAPTER NO. 4 : ANALYSIS COMPETITIVE LANDSCAPE 29
4.1. Company Market Share Analysis – 2024 29
4.1.1. Germany Medical Device Contract Manufacturing Market: Company Market Share, by Volume, 2024 29
4.1.2. Germany Medical Device Contract Manufacturing Market: Company Market Share, by Revenue, 2024 30
4.1.3. Germany Medical Device Contract Manufacturing Market: Top 6 Company Market Share, by Revenue, 2024 30
4.1.4. Germany Medical Device Contract Manufacturing Market: Top 3 Company Market Share, by Revenue, 2024 31
4.2. Germany Medical Device Contract Manufacturing Market Company Revenue Market Share, 2024 32
4.3. Company Assessment Metrics, 2024 33
4.3.1. Stars 33
4.3.2. Emerging Leaders 33
4.3.3. Pervasive Players 33
4.3.4. Participants 33
4.4. Start-ups /SMEs Assessment Metrics, 2024 33
4.4.1. Progressive Companies 33
4.4.2. Responsive Companies 33
4.4.3. Dynamic Companies 33
4.4.4. Starting Blocks 33
4.5. Strategic Developments 34
4.5.1. Acquisitions & Mergers 34
New Product Launch 34
Germany Expansion 34
4.6. Key Players Product Matrix 35
CHAPTER NO. 5 : PESTEL & ADJACENT MARKET ANALYSIS 36
5.1. PESTEL 36
5.1.1. Political Factors 36
5.1.2. Economic Factors 36
5.1.3. Social Factors 36
5.1.4. Technological Factors 36
5.1.5. Environmental Factors 36
5.1.6. Legal Factors 36
5.2. Adjacent Market Analysis 36
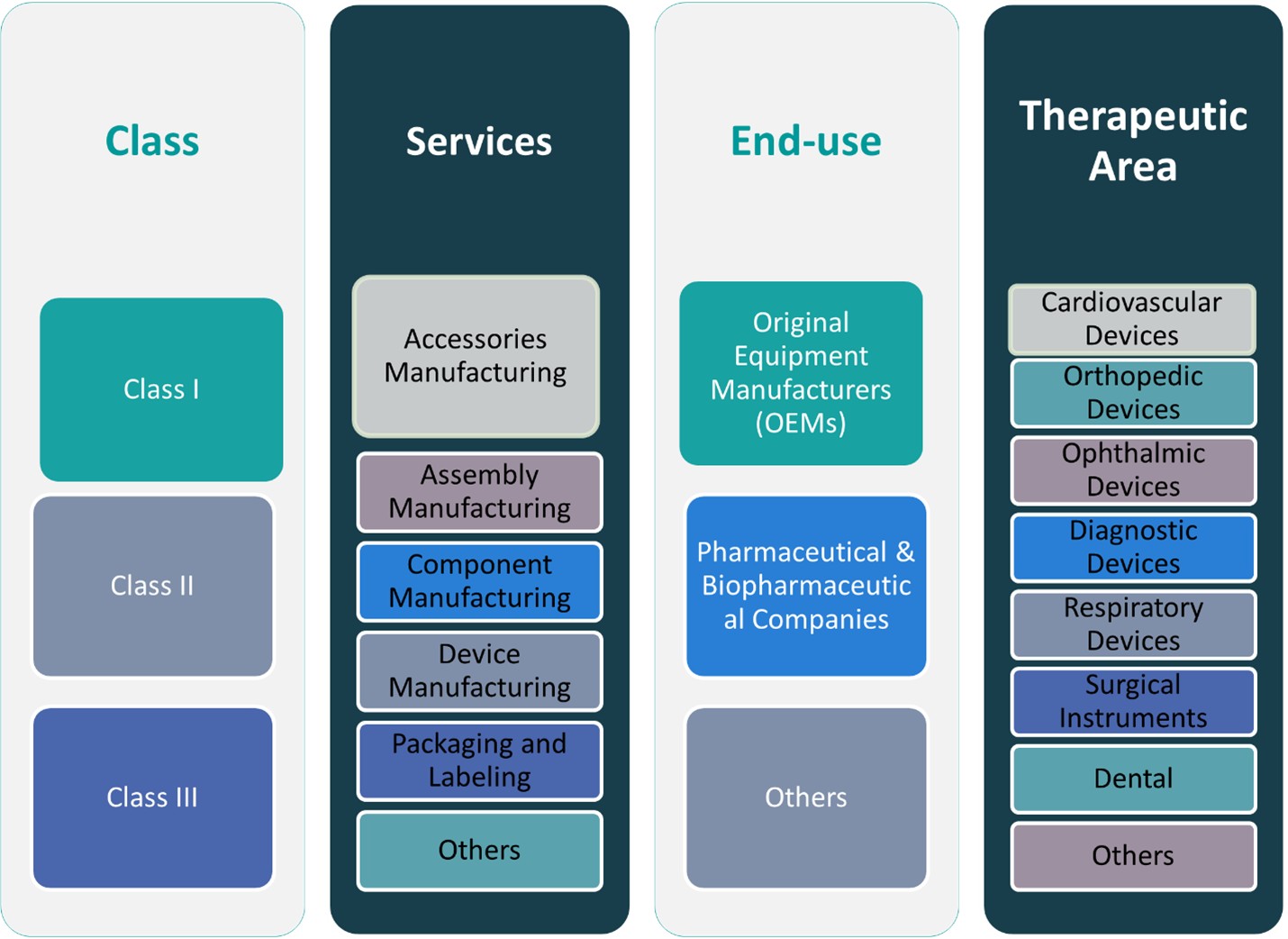
CHAPTER NO. 6 : GERMANY MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – BY CLASS SEGMENT ANALYSIS 37
6.1. Germany Medical Device Contract Manufacturing Market Overview, by Class Segment 37
6.1.1. Germany Medical Device Contract Manufacturing Market Revenue Share, By Class, 2023 & 2032 38
6.1.2. Germany Medical Device Contract Manufacturing Market Attractiveness Analysis, By Class 39
6.1.3. Incremental Revenue Growth Opportunity, by Class, 2024 – 2032 39
6.1.4. Germany Medical Device Contract Manufacturing Market Revenue, By Class, 2018, 2023, 2027 & 2032 40
6.2. Class I 41
6.3. Class II 42
6.4. Class III 43
CHAPTER NO. 7 : GERMANY MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – BY SERVICES SEGMENT ANALYSIS 44
7.1. Germany Medical Device Contract Manufacturing Market Overview, by Services Segment 44
7.1.1. Germany Medical Device Contract Manufacturing Market Revenue Share, By Services, 2023 & 2032 45
7.1.2. Germany Medical Device Contract Manufacturing Market Attractiveness Analysis, By Services 46
7.1.3. Incremental Revenue Growth Opportunity, by Services, 2024 – 2032 46
7.1.4. Germany Medical Device Contract Manufacturing Market Revenue, By Services, 2018, 2023, 2027 & 2032 47
7.2. Accessories Manufacturing 48
7.3. Assembly Manufacturing 49
7.4. Component Manufacturing 50
7.5. Device Manufacturing 51
7.6. Packaging and Labeling 52
7.7. Others 53
CHAPTER NO. 8 : GERMANY MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – BY END-USE SEGMENT ANALYSIS 54
8.1. Germany Medical Device Contract Manufacturing Market Overview, by End-use Segment 54
8.1.1. Germany Medical Device Contract Manufacturing Market Revenue Share, By End-use, 2023 & 2032 55
8.1.2. Germany Medical Device Contract Manufacturing Market Attractiveness Analysis, By End-use 56
8.1.3. Incremental Revenue Growth Opportunity, by End-use, 2024 – 2032 56
8.1.4. Germany Medical Device Contract Manufacturing Market Revenue, By End-use, 2018, 2023, 2027 & 2032 57
8.2. Original Equipment Manufacturers (OEMs) 58
8.3. Pharmaceutical & Biopharmaceutical Companies 59
8.4. Others 60
CHAPTER NO. 9 : GERMANY MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – BY THERAPEUTIC AREA SEGMENT ANALYSIS 61
9.1. Germany Medical Device Contract Manufacturing Market Overview, by Therapeutic Area Segment 61
9.1.1. Germany Medical Device Contract Manufacturing Market Revenue Share, By Therapeutic Area, 2023 & 2032 62
9.1.2. Germany Medical Device Contract Manufacturing Market Attractiveness Analysis, By Therapeutic Area 63
9.1.3. Incremental Revenue Growth Opportunity, by Therapeutic Area, 2024 – 2032 63
9.1.4. Germany Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018, 2023, 2027 & 2032 64
9.2. Cardiovascular Devices 65
9.3. Orthopedic Devices 66
9.4. Ophthalmic Devices 67
9.5. Diagnostic Devices 68
9.6. Respiratory Devices 69
9.7. Surgical Instruments 70
9.8. Dental 71
9.9. Others 72
CHAPTER NO. 10 : GERMANY MEDICAL DEVICE CONTRACT MANUFACTURING MARKET – GERMANY ANALYSIS 73
10.1. Class 73
10.1.1. Germany Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 73
10.1.2. Germany Medical Device Contract Manufacturing Market Revenue, By Class, 2024 – 2032 (USD Million) 73
10.2. Services 74
10.2.1. Germany Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 74
10.2.2. Germany Medical Device Contract Manufacturing Market Revenue, By Services, 2024 – 2032 (USD Million) 74
10.3. End-use 75
10.3.1. Germany Medical Device Contract Manufacturing Market Revenue, By End-use, 2018 – 2023 (USD Million) 75
10.3.2. Germany Medical Device Contract Manufacturing Market Revenue, By End-use, 2024 – 2032 (USD Million) 75
10.4. Therapeutic Area 76
10.4.1. Germany Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 76
10.4.2. Germany Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2024 – 2032 (USD Million) 76
CHAPTER NO. 11 : COMPANY PROFILES 77
11.1. Medtronic 77
11.1.1. Company Overview 77
11.1.2. Product Portfolio 77
11.1.3. Swot Analysis 77
11.1.4. Business Strategy 78
11.1.5. Financial Overview 78
11.2. Vention Medical 79
11.3. Sartorius Stedim Biotech 79
11.4. Fiedler GmbH 79
11.5. Hapman GmbH 79
11.6. Company 6 79
11.7. Company 7 79
11.8. Company 8 79
11.9. Company 9 79
11.10. Company 10 79
11.11. Company 11 79
11.12. Company 12 79
11.13. Company 13 79
11.14. Company 14 79
List of Figures
FIG NO. 1. Germany Medical Device Contract Manufacturing Market Revenue, 2018 – 2032 (USD Million) 22
FIG NO. 2. Porter’s Five Forces Analysis for Germany Medical Device Contract Manufacturing Market 28
FIG NO. 3. Company Share Analysis, 2024 29
FIG NO. 4. Company Share Analysis, 2024 30
FIG NO. 5. Company Share Analysis, 2024 30
FIG NO. 6. Company Share Analysis, 2024 31
FIG NO. 7. Germany Medical Device Contract Manufacturing Market – Company Revenue Market Share, 2024 32
FIG NO. 8. Germany Medical Device Contract Manufacturing Market Revenue Share, By Class, 2023 & 2032 38
FIG NO. 9. Market Attractiveness Analysis, By Class 39
FIG NO. 10. Incremental Revenue Growth Opportunity by Class, 2024 – 2032 39
FIG NO. 11. Germany Medical Device Contract Manufacturing Market Revenue, By Class, 2018, 2023, 2027 & 2032 40
FIG NO. 12. Germany Medical Device Contract Manufacturing Market for Class I, Revenue (USD Million) 2018 – 2032 41
FIG NO. 13. Germany Medical Device Contract Manufacturing Market for Class II, Revenue (USD Million) 2018 – 2032 42
FIG NO. 14. Germany Medical Device Contract Manufacturing Market for Class III, Revenue (USD Million) 2018 – 2032 43
FIG NO. 15. Germany Medical Device Contract Manufacturing Market Revenue Share, By Services, 2023 & 2032 45
FIG NO. 16. Market Attractiveness Analysis, By Services 46
FIG NO. 17. Incremental Revenue Growth Opportunity by Services, 2024 – 2032 46
FIG NO. 18. Germany Medical Device Contract Manufacturing Market Revenue, By Services, 2018, 2023, 2027 & 2032 47
FIG NO. 19. Germany Medical Device Contract Manufacturing Market for Accessories Manufacturing, Revenue (USD Million) 2018 – 2032 48
FIG NO. 20. Germany Medical Device Contract Manufacturing Market for Assembly Manufacturing, Revenue (USD Million) 2018 – 2032 49
FIG NO. 21. Germany Medical Device Contract Manufacturing Market for Component Manufacturing, Revenue (USD Million) 2018 – 2032 50
FIG NO. 22. Germany Medical Device Contract Manufacturing Market for Device Manufacturing, Revenue (USD Million) 2018 – 2032 51
FIG NO. 23. Germany Medical Device Contract Manufacturing Market for Packaging and Labeling, Revenue (USD Million) 2018 – 2032 52
FIG NO. 24. Germany Medical Device Contract Manufacturing Market for Others, Revenue (USD Million) 2018 – 2032 53
FIG NO. 25. Germany Medical Device Contract Manufacturing Market Revenue Share, By End-use, 2023 & 2032 55
FIG NO. 26. Market Attractiveness Analysis, By End-use 56
FIG NO. 27. Incremental Revenue Growth Opportunity by End-use, 2024 – 2032 56
FIG NO. 28. Germany Medical Device Contract Manufacturing Market Revenue, By End-use, 2018, 2023, 2027 & 2032 57
FIG NO. 29. Germany Medical Device Contract Manufacturing Market for Original Equipment Manufacturers (OEMs), Revenue (USD Million) 2018 – 2032 58
FIG NO. 30. Germany Medical Device Contract Manufacturing Market for Pharmaceutical & Biopharmaceutical Companies, Revenue (USD Million) 2018 – 2032 59
FIG NO. 31. Germany Medical Device Contract Manufacturing Market for Others, Revenue (USD Million) 2018 – 2032 60
FIG NO. 32. Germany Medical Device Contract Manufacturing Market Revenue Share, By Therapeutic Area, 2023 & 2032 62
FIG NO. 33. Market Attractiveness Analysis, By Therapeutic Area 63
FIG NO. 34. Incremental Revenue Growth Opportunity by Therapeutic Area, 2024 – 2032 63
FIG NO. 35. Germany Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018, 2023, 2027 & 2032 64
FIG NO. 36. Germany Medical Device Contract Manufacturing Market for Cardiovascular Devices, Revenue (USD Million) 2018 – 2032 65
FIG NO. 37. Germany Medical Device Contract Manufacturing Market for Orthopedic Devices, Revenue (USD Million) 2018 – 2032 66
FIG NO. 38. Germany Medical Device Contract Manufacturing Market for Ophthalmic Devices, Revenue (USD Million) 2018 – 2032 67
FIG NO. 39. Germany Medical Device Contract Manufacturing Market for Diagnostic Devices, Revenue (USD Million) 2018 – 2032 68
FIG NO. 40. Germany Medical Device Contract Manufacturing Market for Respiratory Devices, Revenue (USD Million) 2018 – 2032 69
FIG NO. 41. Germany Medical Device Contract Manufacturing Market for Surgical Instruments, Revenue (USD Million) 2018 – 2032 70
FIG NO. 42. Germany Medical Device Contract Manufacturing Market for Dental, Revenue (USD Million) 2018 – 2032 71
FIG NO. 43. Germany Medical Device Contract Manufacturing Market for Others, Revenue (USD Million) 2018 – 2032 72
List of Tables
TABLE NO. 1. : Germany Medical Device Contract Manufacturing Market: Snapshot 21
TABLE NO. 2. : Drivers for the Germany Medical Device Contract Manufacturing Market: Impact Analysis 24
TABLE NO. 3. : Restraints for the Germany Medical Device Contract Manufacturing Market: Impact Analysis 26
TABLE NO. 4. : Germany Medical Device Contract Manufacturing Market Revenue, By Class, 2018 – 2023 (USD Million) 73
TABLE NO. 5. : Germany Medical Device Contract Manufacturing Market Revenue, By Class, 2024 – 2032 (USD Million) 73
TABLE NO. 6. : Germany Medical Device Contract Manufacturing Market Revenue, By Services, 2018 – 2023 (USD Million) 74
TABLE NO. 7. : Germany Medical Device Contract Manufacturing Market Revenue, By Services, 2024 – 2032 (USD Million) 74
TABLE NO. 8. : Germany Medical Device Contract Manufacturing Market Revenue, By End-use, 2018 – 2023 (USD Million) 75
TABLE NO. 9. : Germany Medical Device Contract Manufacturing Market Revenue, By End-use, 2024 – 2032 (USD Million) 75
TABLE NO. 10. : Germany Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2018 – 2023 (USD Million) 76
TABLE NO. 11. : Germany Medical Device Contract Manufacturing Market Revenue, By Therapeutic Area, 2024 – 2032 (USD Million) 76