Market Overview:
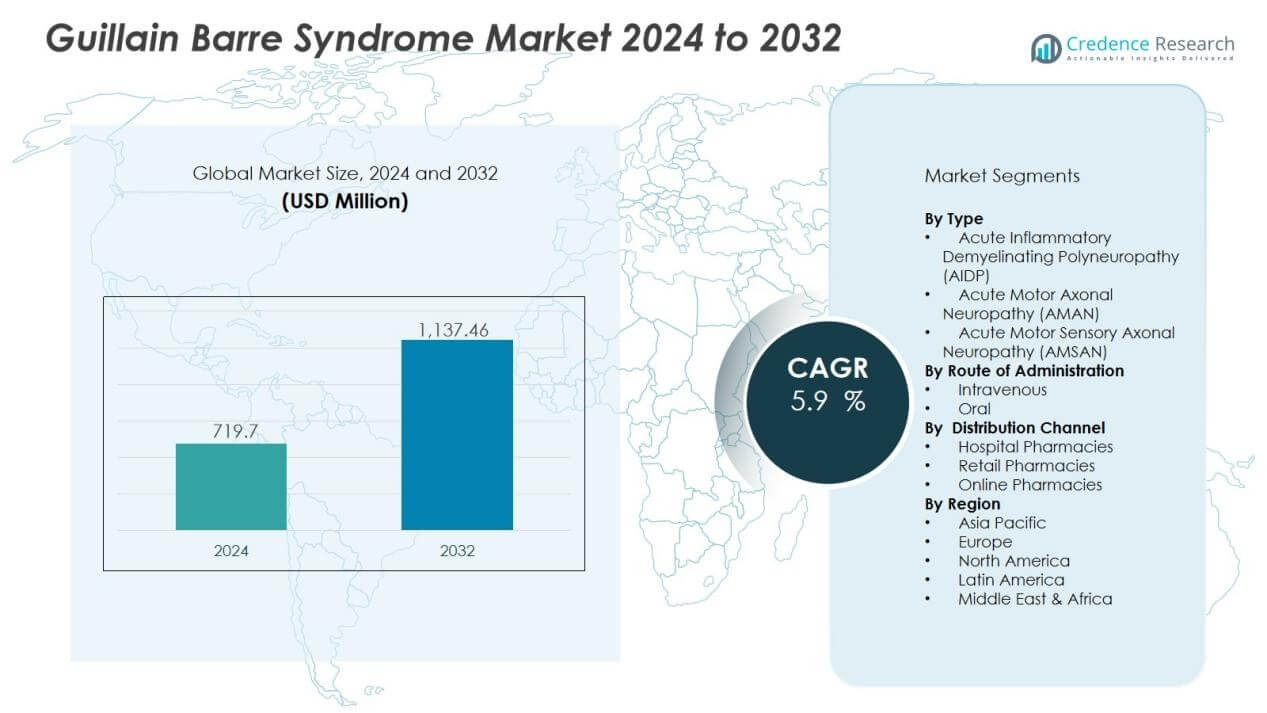
The guillain barre syndrome market size was valued at USD 719.7 million in 2024 and is anticipated to reach USD 1,137.46 million by 2032, at a CAGR of 5.9 % during the forecast period (2024-2032).
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Guillain Barre Syndrome Market Size 2024 |
USD 719.7 Million |
| Guillain Barre Syndrome Market, CAGR |
5.9% |
| Guillain Barre Syndrome Market Size 2032 |
USD 1,137.46 Million |
Key drivers of the Guillain-Barré Syndrome market include the growing incidence of neurological disorders and autoimmune diseases, as well as the rising aging population, which is more vulnerable to such conditions. Demand is further boosted by the availability of immunoglobulin therapies, plasma exchange treatments, and supportive care options. Ongoing clinical trials and R&D efforts by pharmaceutical companies are also fueling innovation, while government initiatives for rare disease management enhance accessibility to therapies.
Regionally, North America dominates the Guillain-Barré Syndrome market due to advanced healthcare infrastructure, high awareness, and strong presence of biopharma companies. Europe follows, supported by favorable reimbursement policies and strong clinical research activities. The Asia-Pacific region is expected to record the fastest growth, driven by expanding healthcare access, rising patient awareness, and government efforts to strengthen rare disease treatment programs in countries like China and India.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights:
- The guillain barre syndrome market was valued at USD 719.7 million in 2024 and is expected to reach USD 1,137.46 million by 2032, growing at a CAGR of 5.9%.
- Rising prevalence of autoimmune and neurological disorders continues to fuel demand for advanced therapeutic interventions.
- Strong adoption of intravenous immunoglobulin therapy and plasma exchange treatments drives market expansion.
- Government-backed rare disease programs and funding initiatives enhance access to costly therapies.
- High treatment costs and limited reimbursement in certain regions remain key barriers to adoption.
- North America held 42% share in 2024, driven by advanced healthcare systems and strong biopharma presence.
- Asia-Pacific accounted for 19% share in 2024 and is projected to grow fastest with expanding healthcare infrastructure and awareness programs.
Market Drivers:
Rising Prevalence of Autoimmune and Neurological Disorders:
The guillain barre syndrome market is driven by the increasing prevalence of autoimmune and neurological disorders worldwide. The growing number of reported cases each year highlights the need for advanced therapeutic interventions. Healthcare providers prioritize early diagnosis and effective treatment to reduce mortality and long-term complications. This trend strongly supports sustained demand for both plasma exchange therapies and immunoglobulin treatments.
- For instance, South Korea documented a significant increase in Guillain-Barré syndrome cases from 648 new diagnoses in 2010 to 941 cases in 2016, representing a population-adjusted incidence rate increase from 1.28 to 1.82 per 100,000 population.
Advancements in Treatment Options and Clinical Research:
It benefits from continuous advancements in treatment methods, including intravenous immunoglobulin therapy and plasma exchange. Ongoing clinical research efforts are introducing new protocols that improve patient recovery outcomes. Pharmaceutical companies are also investing in novel drugs designed to target underlying immune dysfunctions. The availability of advanced treatment options significantly enhances market growth and patient care standards.
- For instance, GC Biopharma launched ALYGLO (immune globulin intravenous, human-stwk) in September 2024, which utilizes an innovative Cation Exchange Chromatography manufacturing process that removes coagulation factor XIa to undetectable levels, demonstrating a 0.03 acute serious bacterial infections per patient-year rate in clinical trials.
Government Support and Rare Disease Management Programs:
Strong government initiatives and rare disease management programs play a vital role in market expansion. Funding for research and patient assistance programs improves access to costly therapies. Awareness campaigns also ensure earlier diagnosis and intervention across healthcare systems. With structured support, the guillain barre syndrome market gains stability and stronger adoption of advanced solutions.
Improved Healthcare Infrastructure and Diagnostic Capabilities:
Expanding healthcare infrastructure and better diagnostic facilities support faster identification of Guillain-Barré Syndrome. Hospitals and specialty centers are equipping themselves with advanced technologies to manage complex neurological conditions. Early and accurate diagnosis enables timely therapeutic intervention, which reduces long-term patient disability. This environment strengthens trust in healthcare systems and fuels consistent market growth.
Market Trends:
Increasing Focus on Advanced Therapeutics and Research Collaborations:
The guillain barre syndrome market is experiencing a shift toward advanced therapeutics supported by strong research collaborations. Pharmaceutical and biotechnology companies are partnering with academic institutions to accelerate clinical trials and improve drug efficacy. Focus is expanding beyond conventional plasma exchange and intravenous immunoglobulin therapies to explore monoclonal antibodies and other targeted treatments. Patient-centric research models encourage personalized approaches to therapy, which align with global healthcare priorities. Continuous investment in neurology-focused R&D strengthens the innovation pipeline and expands long-term growth opportunities. It reflects an industry-wide commitment to improving recovery outcomes and reducing disease burden.
- For Instance, Johnson & Johnson’s subsidiary Janssen announced positive results from a Phase 2 trial for nipocalimab in patients with early-onset severe hemolytic disease of the fetus and newborn (HDFN). The Phase 2 UNITY trial included 13 pregnant individuals at high risk for HDFN.
Expansion of Awareness Programs and Digital Health Integration:
The market is witnessing growing awareness programs that focus on early detection and effective management. Governments and non-profit organizations are investing in educational campaigns that highlight the symptoms and risks of Guillain-Barré Syndrome. Integration of digital health technologies, including telemedicine and AI-driven diagnostic tools, supports faster and more accessible care. Healthcare systems are adopting these digital platforms to monitor patient progress and enhance clinical decision-making. Rising awareness and better digital access lead to higher diagnosis rates and wider adoption of therapies. The guillain barre syndrome market benefits significantly from these combined efforts, ensuring broader reach and improved patient outcomes.
- For Instance, The GBS|CIDP Foundation International launched a global awareness campaign focused on healthcare professionals in May 2025. As part of its mission to advance knowledge of GBS and CIDP, the Foundation has also awarded over $8 million in research grants throughout its history.
Market Challenges Analysis:
High Treatment Costs and Limited Accessibility to Therapies:
The guillain barre syndrome market faces significant challenges due to the high cost of therapies such as intravenous immunoglobulin and plasma exchange. These treatments place a financial burden on patients, especially in regions with limited reimbursement systems. Access to advanced care remains unequal, with rural and low-income areas struggling to provide adequate support. Limited healthcare budgets in developing nations restrict the adoption of innovative treatments. It creates a gap in patient outcomes between high-income and resource-constrained countries.
Diagnostic Delays and Shortage of Specialized Healthcare Professionals:
Delayed diagnosis continues to hinder effective management of Guillain-Barré Syndrome across many regions. The lack of awareness among patients and primary care providers often leads to late referrals. A shortage of specialized neurologists further complicates timely intervention. Misdiagnosis risks remain high, given the overlapping symptoms with other neurological disorders. The guillain barre syndrome market must address these structural barriers to achieve better outcomes. It requires stronger training programs and wider availability of diagnostic tools to reduce delays and improve treatment success.
Market Opportunities:
Growing Investment in Novel Therapeutics and Research Initiatives:
The guillain barre syndrome market offers strong opportunities through growing investments in novel therapeutics and research programs. Pharmaceutical companies are directing resources toward monoclonal antibodies, stem cell therapies, and next-generation immunomodulators. These advancements aim to reduce recovery times and lower the risk of long-term disability. Expanding clinical trial networks create pathways for faster approvals and broader patient participation. Governments and private organizations are also providing funding to accelerate innovation. It creates a favorable environment for the introduction of breakthrough therapies that can transform patient outcomes.
Expanding Reach in Emerging Healthcare Markets:
The market is well-positioned to benefit from the expansion of healthcare infrastructure in developing regions. Countries in Asia-Pacific, Latin America, and the Middle East are increasing investments in neurology care and rare disease management. Rising awareness campaigns and improved access to diagnostic facilities drive earlier intervention. Affordable treatment models and public-private partnerships further support accessibility for underrepresented populations. Telemedicine platforms and digital tools also improve patient monitoring and follow-up in resource-limited settings. The guillain barre syndrome market gains new growth avenues by capitalizing on these expanding opportunities.
Market Segmentation Analysis:
By Type:
The guillain barre syndrome market is segmented into acute inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy (AMAN), and acute motor sensory axonal neuropathy (AMSAN). AIDP holds the largest share due to its higher prevalence across North America and Europe. AMAN and AMSAN are more frequently reported in Asia-Pacific, supported by growing awareness and diagnostic improvements. It reflects regional variations in clinical presentation and treatment approaches.
- For Instance, The first comprehensive nationwide study on GBS incidence in mainland China, covering 2016 to 2019, was published in ScienceDirect. This study, which involved 38,861 GBS patients, found the overall incidence to be 0.698 per 100,000 person-years.
By Route of Administration:
The market is categorized into intravenous and oral routes of administration. Intravenous immunoglobulin therapy dominates this segment because of its established effectiveness in managing symptoms and improving recovery. Plasma exchange treatments also rely heavily on hospital-based intravenous procedures. Oral therapies remain limited but represent an area of interest for future drug development. It continues to show preference for intravenous delivery due to faster action and clinical reliability.
- For instance, Takeda’s GAMMAGARD LIQUID received FDA approval in January 2024 for CIDP treatment, demonstrating a 94.4% responder rate with an improvement in functional disability in clinical trials.
By Distribution Channel:
Distribution channels include hospital pharmacies, retail pharmacies, and online platforms. Hospital pharmacies lead due to the need for supervised administration of intravenous immunoglobulin and plasma exchange therapies. Retail pharmacies play a supporting role in providing supportive medications and post-care drugs. Online platforms are gradually gaining traction in regions with improved digital health ecosystems. It demonstrates stronger reliance on hospital-based channels, while digital models open new opportunities for accessibility.
Segmentations:
By Type:
- Acute Inflammatory Demyelinating Polyneuropathy (AIDP)
- Acute Motor Axonal Neuropathy (AMAN)
- Acute Motor Sensory Axonal Neuropathy (AMSAN)
By Route of Administration:
By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
By Region:
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
North America:
North America accounted for 42% market share in 2024, making it the largest regional contributor. The region benefits from advanced healthcare systems, high awareness levels, and strong reimbursement policies. The guillain barre syndrome market in the U.S. is supported by the presence of major biopharmaceutical companies driving innovation. Government programs and rare disease initiatives ensure wider access to treatments such as intravenous immunoglobulin and plasma exchange. Strong clinical research capacity across the region accelerates development of novel therapeutics. It continues to dominate due to robust investment in neurology-focused healthcare infrastructure and early adoption of advanced therapies.
Europe:
Europe held 28% market share in 2024, supported by favorable regulatory frameworks and active research collaborations. Countries such as Germany, France, and the U.K. are at the forefront of adopting advanced therapies for Guillain-Barré Syndrome. The guillain barre syndrome market in this region benefits from strong rare disease policies and government-funded awareness programs. Clinical trials in Europe continue to focus on immunotherapy development, offering new treatment prospects. Reimbursement systems provide significant support for patients, reducing financial barriers to care. It is expected to sustain its position with continuous investments in neurology and biotechnology research.
Asia-Pacific:
Asia-Pacific accounted for 19% market share in 2024, with the fastest projected growth rate through 2032. Rising healthcare expenditure in China, India, and Japan supports wider access to advanced therapies. The guillain barre syndrome market here is driven by government initiatives aimed at strengthening rare disease care. Expanding diagnostic infrastructure and telemedicine services improve early detection and timely treatment. Patient awareness campaigns supported by public-private partnerships boost early referrals and adoption of therapies. It is expected to expand rapidly as regional healthcare systems continue to prioritize neurological and autoimmune disease management.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- AbbVie Inc.
- Cadila Pharmaceuticals
- Biogen
- F. Hoffmann-La Roche Ltd.
- CSL
- GSK plc
- LGM Pharma
- Merck & Co., Inc.
- Grifols S.A.
- Octapharma AG
- Pfizer Inc.
Competitive Analysis:
The guillain barre syndrome market is shaped by the presence of global pharmaceutical leaders and regional players. Key companies include AbbVie Inc., Cadila Pharmaceuticals, Biogen, F. Hoffmann-La Roche Ltd., CSL, GSK plc, LGM Pharma, Merck & Co., Inc., and Grifols S.A. These firms focus on immunoglobulin therapies, plasma exchange technologies, and pipeline innovations to improve recovery outcomes. Competitive intensity remains high as companies expand research collaborations and clinical trial activities. Strategic investments in rare disease treatment portfolios strengthen long-term positioning and global reach. It is witnessing an increase in partnerships between biotech firms and healthcare institutions, aiming to accelerate novel therapeutic development. Established players maintain market dominance through strong distribution networks and regulatory expertise, while emerging firms target niche opportunities with innovative approaches.
Recent Developments:
- In August 2025, AbbVie announced the acquisition of Capstan Therapeutics for up to $2.1 billion, gaining access to Capstan’s lead clinical candidate CPTX2309 and their proprietary in vivo cell engineering technology.
- In June 2025, Cadila Pharmaceuticals launched Biscado, a beta-1 adrenergic blocker for treating cardiovascular diseases including hypertension and chronic heart failure.
Report Coverage:
The research report offers an in-depth analysis based on Type, Route of Administration, Distribution Channel and Region. It details leading Market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current Market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven Market expansion in recent years. The report also explores Market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on Market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the Market.
Future Outlook:
- The guillain barre syndrome market will see stronger growth through continued innovation in therapeutic approaches.
- Rising adoption of monoclonal antibodies and next-generation immunotherapies will redefine treatment standards.
- It will benefit from faster clinical trial approvals and broader participation across global patient groups.
- Government-backed rare disease programs will expand funding support and patient access to advanced therapies.
- Digital health tools and telemedicine will play a larger role in patient monitoring and follow-up.
- Increased collaboration between pharmaceutical firms and research institutions will accelerate the discovery of novel treatments.
- The market will experience greater penetration in emerging regions with expanding healthcare infrastructure.
- Improved diagnostic technologies will reduce delays and ensure timely therapeutic interventions for patients.
- It will witness stronger focus on personalized medicine, enabling tailored treatment approaches for diverse patient profiles.
- Long-term market stability will be shaped by sustained investment in R&D and global policy support.




