Market Overview
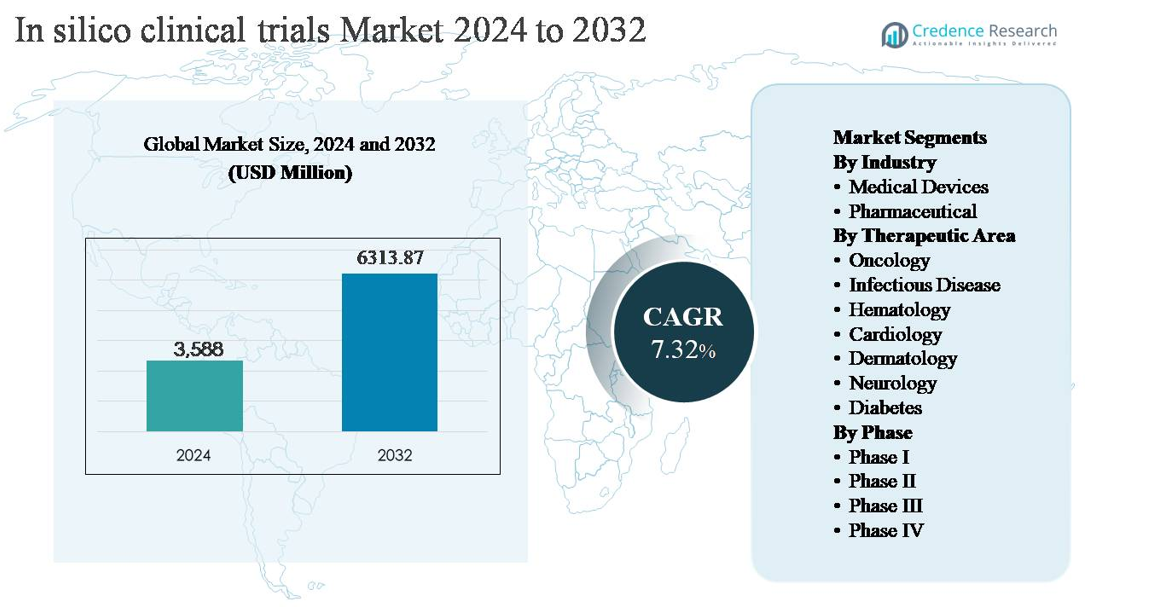
The in silico clinical trials market was valued at USD 3,588 million in 2024 and is projected to reach USD 6,313.87 million by 2032, expanding at a compound annual growth rate (CAGR) of 7.32% over the forecast period.
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| In Silico Clinical Trials Market Size 2024 |
USD 3,588 million |
| In Silico Clinical Trials Market, CAGR |
7.32% |
| In Silico Clinical Trials Market Size 2032 |
USD 6,313.87 million |
The in silico clinical trials market is led by a group of specialized technology and life-science analytics companies that provide advanced modeling, simulation, and AI-driven platforms. Key players such as Dassault Systèmes SE, Certara, Inc., Insilico Medicine, Inc., GNS Healthcare Inc., Novadiscovery SAS, InSilicoTrials, Immunetrics Inc., Nuventra Pharma Sciences, Abzena Ltd., and The AnyLogic Company compete on predictive accuracy, regulatory-aligned validation, and integration across drug and device development workflows. These companies support pharmaceutical and medical device sponsors in optimizing trial design, dose selection, and patient stratification. North America remains the leading regional market, accounting for approximately 38% of the global market share, driven by strong R&D investment, early technology adoption, and supportive regulatory engagement.
Market Insights
- The in silico clinical trials market was valued at USD 3,588 million in 2024 and is projected to reach USD 6,313.87 million by 2032, growing at a CAGR of 7.32% during the forecast period.
- Market growth is primarily driven by rising costs and complexity of conventional clinical trials, pushing pharmaceutical and medical device companies to adopt simulation-based approaches to reduce development timelines, optimize protocols, and improve trial success rates, with the pharmaceutical segment holding the dominant share due to extensive drug R&D activity.
- Key market trends include increasing integration of AI, machine learning, and digital twin technologies, alongside expanding use in oncology and cardiology, where oncology remains the leading therapeutic area segment due to high trial failure risks and demand for personalized treatment modeling.
- The competitive landscape is marked by specialized players offering advanced modeling platforms, with competition focused on predictive accuracy, regulatory acceptance, and end-to-end workflow integration through strategic collaborations with life science companies.
- Regionally, North America leads with approximately 38% market share, followed by Europe at 28% and Asia Pacific at 22%, while Latin America and Middle East & Africa collectively account for the remaining share, reflecting emerging adoption.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Segmentation Analysis:
By Industry:
The pharmaceutical segment represents the dominant share of the in silico clinical trials market, accounting for the majority of overall revenue due to its extensive use across drug discovery and clinical development pipelines. Pharmaceutical companies increasingly deploy in silico modeling to optimize dose selection, predict efficacy, and reduce late-stage trial failures, particularly in complex biologics and small-molecule programs. The medical devices segment, while smaller, is expanding steadily as regulatory agencies accept computational evidence for device safety and performance. The dominance of pharmaceuticals is driven by high R&D intensity, escalating trial costs, and strong pressure to shorten development timelines.
- For instance, Novartis has publicly documented the use of QSP platforms to simulate drug-disease interactions across virtual cohorts exceeding 10,000 modeled patients for immunology and oncology programs, enabling evaluation of multiple dose regimens and schedules before first-in-human studies.
By Therapeutic Area:
Oncology is the dominant therapeutic area in the in silico clinical trials market, holding the largest market share due to the high complexity of cancer biology and the need for personalized treatment modeling. Virtual tumor simulations, biomarker stratification, and response prediction tools are widely used to optimize trial design and patient selection. Infectious disease follows, supported by outbreak modeling and vaccine development applications, while cardiology and neurology show rising adoption through organ-level simulations. Oncology leadership is driven by high clinical failure rates, precision medicine demand, and substantial oncology R&D spending.
- For instance, Roche and its Genentech division have reported the use of quantitative systems pharmacology models integrating genomic and pharmacology data from more than 11,000 tumor samples derived from The Cancer Genome Atlas to simulate pathway inhibition and predict response variability across virtual oncology cohorts.
By Phase:
Phase II constitutes the dominant phase segment in the in silico clinical trials market, capturing the highest market share as sponsors increasingly rely on simulations to evaluate efficacy signals and refine trial endpoints. In silico approaches are particularly valuable in Phase II for reducing patient numbers and improving go/no-go decisions before costly late-stage trials. Phase I adoption is supported by safety and dose-finding simulations, while Phase III usage is growing for virtual cohort supplementation. The dominance of Phase II is driven by its critical role in attrition reduction and cost optimization.
Key Growth Drivers
Rising Cost and Complexity of Conventional Clinical Trials
Escalating costs, prolonged timelines, and high failure rates in traditional clinical trials are a primary driver accelerating adoption of in silico clinical trials. Late-stage clinical studies require large patient cohorts, multi-site coordination, and extensive regulatory oversight, significantly increasing development expenditures. In silico approaches enable sponsors to simulate patient responses, refine inclusion criteria, and optimize dosing strategies before physical trials begin. These capabilities help reduce unnecessary protocol amendments and lower patient recruitment burdens. By identifying ineffective candidates earlier, developers can redirect resources more efficiently. The growing pressure on pharmaceutical and medical device companies to control R&D spending while maintaining innovation output continues to push in silico trials into mainstream development strategies.
- For instance, AstraZeneca has disclosed the use of model-informed drug development frameworks that integrate PBPK and disease-progression models to simulate clinical outcomes across virtual cohorts exceeding 5,000 patients, enabling protocol refinement and dose optimization prior to Phase III trial initiation.
Advancements in Computational Modeling and Artificial Intelligence
Rapid improvements in computational biology, machine learning, and digital twin technologies are significantly expanding the scope and reliability of in silico clinical trials. Advanced algorithms can now integrate genomic, proteomic, and physiological datasets to create highly predictive virtual patient populations. These models allow researchers to test multiple trial scenarios simultaneously, improving accuracy in outcome prediction. AI-driven platforms also enhance adaptive trial designs by continuously learning from incoming data. As modeling precision improves, regulatory confidence in simulation-based evidence is strengthening. The convergence of high-performance computing and AI is enabling broader application of in silico methods across therapeutic areas and trial phases.
- For instance, Siemens Healthineers has advanced digital twin technologies through its computational heart models, which are built using millions of finite elements to simulate cardiac electrophysiology and hemodynamics under diverse pathological conditions, supporting virtual evaluation of cardiovascular therapies and interventions.
Growing Regulatory Acceptance and Supportive Frameworks
Regulatory agencies are increasingly recognizing in silico evidence as a complementary component of clinical evaluation, driving market growth. Regulatory guidance now encourages the use of computational modeling for device validation, dose optimization, and risk assessment. In silico trials help support regulatory submissions by providing robust mechanistic insights and reducing reliance on extensive human testing. This shift is particularly evident in medical device development, where virtual testing can demonstrate safety across multiple scenarios. As regulators continue to formalize standards for model validation and reporting, industry confidence in simulation-driven development is increasing, further accelerating adoption.
Key Trends & Opportunities
Expansion of Personalized and Precision Medicine Applications
In silico clinical trials are increasingly aligned with the growth of personalized medicine, creating significant opportunities for market expansion. Virtual patient modeling enables stratification based on genetic, phenotypic, and biomarker data, supporting more targeted therapeutic development. This approach allows developers to simulate treatment responses across diverse subpopulations, improving trial efficiency and outcomes. Precision modeling is especially valuable in oncology and rare diseases, where patient heterogeneity limits traditional trial designs. As personalized therapies gain traction, demand for sophisticated in silico platforms capable of individualized response prediction is expected to rise steadily.
- For instance, Moderna has leveraged in silico neoantigen selection platforms for its personalized cancer vaccine programs, using computational pipelines to prioritize up to 34 patient-specific neoantigen sequences per individual tumor profile prior to clinical manufacturing.
Integration with Real-World Data and Digital Health Technologies
The integration of real-world data from electronic health records, wearable devices, and digital health platforms represents a key growth opportunity for in silico clinical trials. Combining simulation models with real-world evidence enhances predictive accuracy and supports post-market surveillance and adaptive trial designs. This trend enables continuous model refinement and long-term outcome assessment beyond controlled trial settings. Pharmaceutical sponsors increasingly view this integration as a pathway to improve regulatory submissions and lifecycle management strategies. The expanding availability of structured healthcare data is expected to further strengthen this opportunity.
- For instance, Verily’s Project Baseline combined wearable sensor outputs, clinical measurements, and EHR data from 10,000 enrolled participants to generate deeply phenotyped longitudinal datasets used for disease progression modeling and virtual cohort generation.
Key Challenges
Model Validation, Standardization, and Data Quality Limitations
Ensuring the accuracy, reproducibility, and clinical relevance of in silico models remains a major challenge. Variability in data sources, assumptions, and modeling techniques can lead to inconsistent outcomes across platforms. Lack of standardized validation frameworks complicates regulatory review and limits cross-study comparability. High-quality, representative datasets are essential for reliable simulations, yet access to comprehensive clinical and biological data remains uneven. Addressing these challenges requires collaboration between industry, regulators, and technology providers to establish common standards and best practices.
Limited Technical Expertise and Integration Barriers
The effective implementation of in silico clinical trials requires specialized expertise in computational biology, data science, and clinical research, which many organizations lack. Integrating simulation platforms into existing R&D workflows can be complex and resource-intensive. Resistance to change within established clinical development teams further slows adoption. Additionally, aligning multidisciplinary stakeholders around model-driven decision-making presents organizational challenges. Overcoming these barriers will require targeted workforce development, improved user-friendly platforms, and stronger cross-functional collaboration.
Regional Analysis
North America
North America dominates the in silico clinical trials market, accounting for approximately 38% of the global market share. The region benefits from advanced healthcare infrastructure, strong pharmaceutical R&D investment, and early adoption of computational modeling and AI-driven trial platforms. The United States leads regional growth due to the presence of major pharmaceutical companies, specialized software providers, and academic research institutions actively using virtual patient simulations. Favorable regulatory guidance supporting modeling and simulation in drug and device development further strengthens adoption. High clinical trial costs and strong demand for efficiency continue to reinforce North America’s leadership position.
Europe
Europe holds nearly 28% of the global in silico clinical trials market share, supported by strong regulatory alignment, public–private research collaborations, and growing acceptance of digital evidence in clinical development. Countries such as Germany, the United Kingdom, and France are key contributors, driven by robust biomedical research ecosystems and rising investment in personalized medicine. European regulators increasingly support modeling and simulation for safety assessment and dose optimization, particularly in medical devices. The region’s focus on cost containment, ethical trial design, and innovation in computational biology sustains steady market expansion.
Asia Pacific
Asia Pacific accounts for approximately 22% of the global market share and represents the fastest-growing regional market for in silico clinical trials. Rapid expansion of pharmaceutical manufacturing, increasing clinical trial activity, and rising digital health investments support adoption. China, Japan, South Korea, and India are key markets, driven by growing computational capabilities and expanding biotech sectors. Governments across the region are promoting AI and data-driven healthcare innovation, accelerating use of virtual trial methodologies. Increasing clinical trial volumes and pressure to reduce development timelines continue to drive strong regional growth momentum.
Latin America
Latin America represents around 7% of the global in silico clinical trials market, with gradual adoption driven by expanding clinical research activity and improving healthcare infrastructure. Brazil and Mexico are the primary contributors, supported by growing pharmaceutical outsourcing and regional trial participation. While adoption remains limited compared to developed regions, increasing awareness of cost-efficient trial models is encouraging interest in in silico approaches. Regional growth is supported by collaborations with global sponsors seeking optimized trial designs. Continued investment in digital health capabilities is expected to strengthen market penetration over time.
Middle East & Africa
The Middle East & Africa region holds approximately 5% of the global market share, reflecting early-stage adoption of in silico clinical trials. Growth is supported by healthcare modernization initiatives, particularly in Gulf Cooperation Council countries investing in digital health and advanced research infrastructure. South Africa also contributes through expanding clinical research capabilities. Limited access to high-quality datasets and technical expertise currently constrains adoption. However, increasing participation in global clinical trials and rising interest in AI-driven healthcare solutions are expected to gradually improve regional market presence.
Market Segmentations:
By Industry
- Medical Devices
- Pharmaceutical
By Therapeutic Area
- Oncology
- Infectious Disease
- Hematology
- Cardiology
- Dermatology
- Neurology
- Diabetes
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Geography
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Competitive Landscape
The competitive landscape of the in silico clinical trials market is characterized by a mix of specialized simulation software providers, AI-driven analytics firms, and established life sciences technology companies. Market participants compete on the depth of biological modeling, predictive accuracy, and the ability to integrate multi-omics and real-world data into virtual patient simulations. Leading players focus on expanding platform capabilities across multiple therapeutic areas and clinical phases, while strengthening regulatory-aligned validation frameworks. Strategic collaborations with pharmaceutical companies, medical device manufacturers, and academic institutions are common, enabling co-development of disease-specific models. Vendors also invest heavily in artificial intelligence, cloud-based architectures, and digital twin technologies to enhance scalability and usability. Competitive differentiation increasingly depends on model transparency, regulatory acceptance, and end-to-end workflow integration, positioning innovation and credibility as critical success factors.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis
- Dassault Systemes SE
- Insilico Medicine, Inc.
- Certara, Inc.
- GNS Healthcare Inc.
- Novadiscovery SAS
- InSilicoTrials
- The AnyLogic Company
- Immunetrics Inc.
- Abzena Ltd.
- Nuventra Pharma Sciences
Recent Developments
- In January 2025, Insilico Medicine advanced its AI-enabled in silico clinical trial framework to support Phase II development of INS018_055, an anti-fibrotic small molecule. The company applied multi-modal simulation models trained on over 1 billion biological and chemical data points, integrating transcriptomic signatures and pharmacokinetic parameters to predict dose response and responder stratification prior to patient enrollment. The in silico workflow simulated treatment outcomes across several thousand virtual patients, supporting protocol optimization and biomarker-informed cohort selection.
- In October 2024, Dassault Systèmes expanded its Living Heart Project simulation portfolio to support in silico clinical evidence generation for cardiovascular drugs and devices. The updated platform enables electrophysiology and hemodynamic simulations using cardiac models containing over 5 million finite elements, allowing virtual evaluation of drug-induced arrhythmia risk and devicetissue interactions across patient-specific anatomies. The platform integrates clinical imaging data, including CT and MRI inputs with spatial resolutions below 1 mm, enabling realistic virtual cohorts for regulatory-grade simulation studies.
- In July 2024,Certara released an upgraded version of its Simcyp™ physiologically based pharmacokinetic (PBPK) simulator, widely used for in silico clinical trials and regulatory submissions. The update expanded virtual population libraries to include more than 40 predefined physiological population models, covering pediatric, geriatric, renal impairment, and hepatic impairment scenarios. Each model incorporates over 20 organ compartments with age- and disease-specific physiological parameters, enabling virtual assessment of dosing, drug-drug interactions, and exposure variability before clinical execution.
Report Coverage
The research report offers an in-depth analysis based on Industry, Therapeutic area, Phase and Geography. It details leading market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven market expansion in recent years. The report also explores market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the market.
Future Outlook
- Adoption of in silico clinical trials will continue to expand as sponsors seek to reduce development timelines and improve trial efficiency.
- Computational modeling will play a larger role in early decision-making across drug and medical device development.
- Artificial intelligence and machine learning will further enhance predictive accuracy and adaptive trial design capabilities.
- Regulatory acceptance of simulation-based evidence will strengthen, supporting broader use in submissions and approvals.
- Integration with real-world data will improve long-term outcome prediction and post-market evaluation.
- Personalized medicine initiatives will drive demand for patient-specific and biomarker-driven virtual trials.
- Oncology and cardiology will remain leading therapeutic areas for advanced in silico applications.
- Cloud-based platforms will improve scalability and global accessibility of simulation tools.
- Collaboration between technology providers, pharmaceutical companies, and regulators will intensify.
- Standardization of modeling frameworks will improve credibility, interoperability, and industry-wide adoption.




