Market Overview:
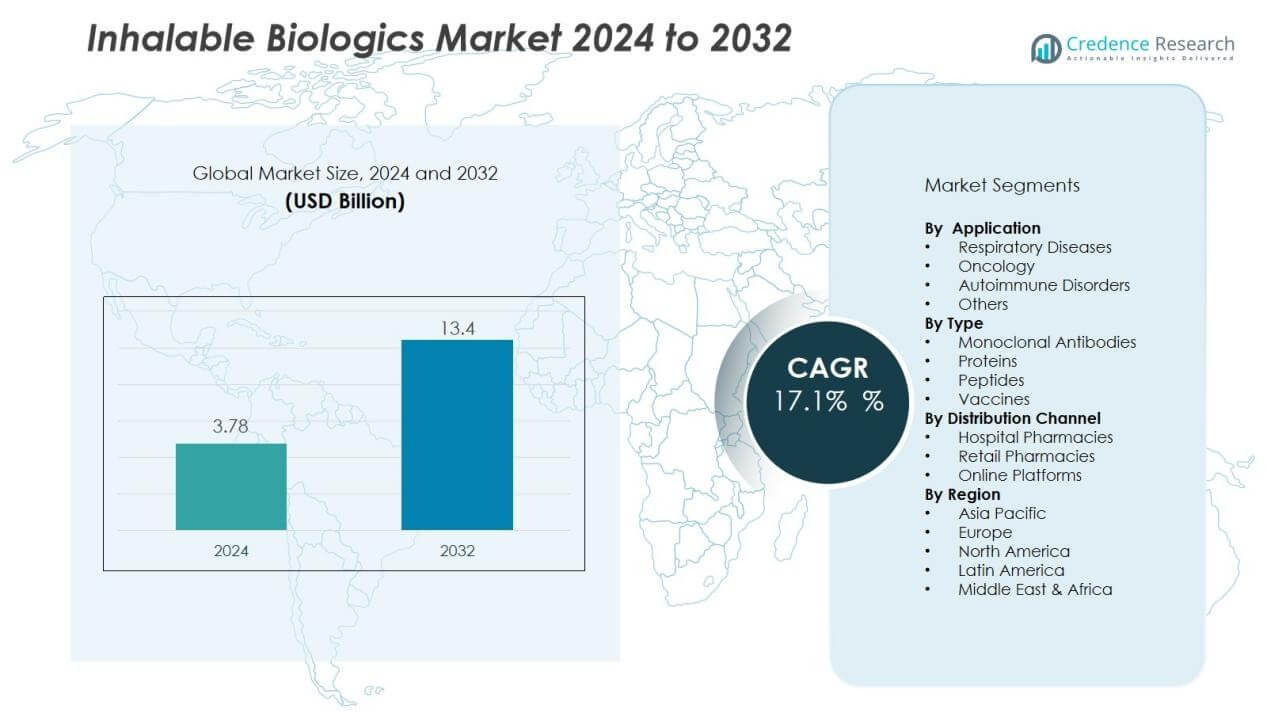
The inhalable biologics market size was valued at USD 3.78 billion in 2024 and is anticipated to reach USD 13.4 billion by 2032, at a CAGR of 17.1% during the forecast period (2024-2032).
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Inhalable Biologics Market Size 2024 |
USD 3.78 Billion |
| Inhalable Biologics Market, CAGR |
17.1% |
| Inhalable Biologics Market Size 2032 |
USD 13.4 Billion |
Growth is driven by increasing prevalence of respiratory diseases, rising focus on patient-friendly delivery options, and expanding applications in chronic conditions like asthma, COPD, and cystic fibrosis. The ability of inhalable biologics to bypass first-pass metabolism enhances treatment efficiency. Pharmaceutical companies are investing in research collaborations and device innovations to meet growing demand. The trend toward non-invasive treatment is also encouraging healthcare providers to adopt inhalable formulations over injectables.
Regionally, North America dominates the inhalable biologics market due to strong healthcare infrastructure, high R&D investments, and early adoption of advanced therapeutics. Europe follows closely, supported by favorable regulatory frameworks and strong presence of biopharma companies. Asia-Pacific is expected to register the fastest growth, driven by a large patient base, expanding clinical trials, and rising healthcare spending. Latin America and the Middle East & Africa are emerging markets with growing opportunities, though they remain limited by infrastructure and affordability challenges.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Insights:
- The inhalable biologics market was valued at USD 3.78 billion in 2024 and is projected to reach USD 13.4 billion by 2032, growing at a CAGR of 17.1%.
- Rising prevalence of asthma, COPD, and cystic fibrosis drives demand for inhalable biologics as effective treatment options.
- Patients and providers prefer non-invasive delivery methods, with inhalable biologics offering needle-free administration and better compliance.
- Advances in biologic formulations, particle engineering, and smart inhalation devices improve drug stability, dosing accuracy, and treatment efficiency.
- High development costs and complex manufacturing remain barriers, with smaller firms facing funding challenges and longer timelines.
- North America leads with 42% share in 2024, followed by Europe at 31%, while Asia Pacific, with 19%, is the fastest-growing region.
- Strong investments in R&D, strategic collaborations, and supportive regulatory frameworks enhance growth potential across multiple therapeutic areas.
Market Drivers:
Rising Prevalence of Respiratory and Chronic Diseases Driving Demand:
The inhalable biologics market benefits from the growing burden of asthma, COPD, cystic fibrosis, and other respiratory disorders. Rising urban pollution, smoking habits, and genetic predispositions contribute to higher patient numbers worldwide. Biologics delivered through inhalation provide targeted therapy and improved disease control. Healthcare systems are adopting such treatments to reduce hospital admissions and long-term costs.
- For instance, Genentech’s Pulmozyme (recombinant human DNase) once-daily inhalation reduced the age-adjusted risk of respiratory exacerbations by 28% in cystic fibrosis patients over 24 weeks.
Preference for Non-Invasive and Patient-Friendly Drug Delivery Methods:
Patients and healthcare providers increasingly prefer delivery methods that reduce discomfort and improve adherence. The inhalable biologics market leverages this shift by offering needle-free administration compared to injectables. Such convenience encourages higher compliance in chronic disease management. Pharmaceutical companies are innovating inhalation devices to improve portability and ease of use.
- For instance, MannKind’s Afrezza rapid-acting inhaled insulin demonstrated a time to first measurable effect of approximately 12 minutes with the 4-unit cartridge in a crossover study of type 1 diabetes patients.
Advancements in Biologic Formulations and Inhalation Technologies:
Continuous progress in biologics manufacturing and inhalation device design strengthens the market. The inhalable biologics market benefits from improved stability, dosing accuracy, and bioavailability. Advances in dry powder inhalers, nebulizers, and smart inhalation systems enhance treatment efficiency. Partnerships between biotech firms and device manufacturers accelerate new product launches and clinical acceptance.
Growing Investment in Research, Development, and Strategic Collaborations:
Pharmaceutical and biotechnology companies are increasing investments to expand inhalable biologics pipelines. It is supported by rising clinical trials targeting respiratory, oncology, and autoimmune conditions. Collaborations with academic institutions and technology firms help accelerate innovation. Favorable regulatory support for biologics further strengthens the growth outlook of this emerging market.
Market Trends:
Integration of Smart Inhalation Devices and Digital Health Platforms:
The inhalable biologics market is experiencing a notable trend toward digital integration. Smart inhalers equipped with sensors and connectivity features are gaining adoption. These devices allow patients and providers to track dosage, monitor adherence, and analyze usage patterns in real time. Digital health platforms link inhalers with mobile applications to improve patient engagement. Pharmaceutical companies are forming partnerships with digital health firms to bring combined therapeutic and monitoring solutions. This trend enhances treatment outcomes while creating opportunities for personalized care delivery.
- For instance, Propeller Health’s sensor-enabled inhaler platform demonstrated a 58% improvement in medication adherence among asthma patients over an 11-month period.
Expansion Beyond Respiratory Disorders Into Oncology and Autoimmune Diseases:
The inhalable biologics market is diversifying its application scope beyond respiratory conditions. Researchers are exploring inhalable biologics for oncology, diabetes, and autoimmune diseases to improve targeted delivery. It is supported by advances in particle engineering, drug stability, and pulmonary absorption research. Several clinical trials are underway to evaluate efficacy in broader therapeutic areas. Biopharma firms are investing in pipeline expansion to capture untapped demand. This expansion demonstrates the potential of inhalable biologics as a versatile platform for future therapies.
- For Instance, Based on results from a pharmacokinetic study and pooled data from multiple clinical trials, MannKind’s Afrezza inhalable insulin has a relative bioavailability of 21–30% and a mean time to maximum concentration (Tmax) of 12–15 minutes.
Market Challenges Analysis:
High Development Costs and Complex Manufacturing Requirements:
The inhalable biologics market faces significant hurdles due to high research and production expenses. Biologics require advanced facilities, strict temperature control, and precise formulation to maintain stability. Inhalation delivery further demands specialized particle engineering and device compatibility. These requirements extend development timelines and raise overall costs for manufacturers. Smaller biotech firms often struggle to secure funding for large-scale projects. It creates barriers to entry and slows the pace of innovation in this market.
Regulatory Uncertainty and Limited Long-Term Clinical Data:
The inhalable biologics market also contends with evolving regulatory frameworks. It must comply with stringent approval processes for both biologic molecules and delivery devices. Limited availability of long-term clinical data raises concerns about safety, efficacy, and patient outcomes. Regulatory agencies often require extensive evidence before granting approvals, leading to delays. Variations in regional guidelines create added complexity for global market expansion. These challenges restrict faster commercialization and reduce confidence among investors and healthcare providers.
Market Opportunities:
Expanding Applications Across Therapeutic Areas and Personalized Medicine:
The inhalable biologics market presents strong opportunities through diversification into new therapeutic areas. Beyond respiratory diseases, researchers are advancing applications in oncology, diabetes, and autoimmune disorders. Inhalation routes provide faster onset of action and better targeting compared to traditional methods. It creates potential for more personalized and effective treatment regimens. The growing interest in precision medicine further supports the use of inhalable biologics for tailored patient care. Companies investing in broad clinical pipelines stand to capture significant value.
Rising Adoption in Emerging Markets and Favorable Healthcare Investments:
The inhalable biologics market is poised to benefit from increasing healthcare spending in emerging economies. Countries in Asia-Pacific, Latin America, and the Middle East are expanding infrastructure and access to advanced therapeutics. Rising patient populations, combined with supportive government initiatives, create strong demand potential. It is supported by pharmaceutical firms forming local partnerships and expanding distribution networks. Advances in low-cost inhalation devices also open doors for wider accessibility. These factors position emerging markets as key growth hubs for global expansion.
Market Segmentation Analysis:
By Type:
The inhalable biologics market by type includes monoclonal antibodies, proteins, peptides, and vaccines. Monoclonal antibodies lead due to their effectiveness in treating chronic and respiratory diseases. Proteins and peptides are gaining traction with rising demand for targeted therapies. It benefits from ongoing innovations in particle formulation and inhalation device compatibility. Vaccines delivered via inhalation show promise for respiratory infections, offering painless administration and improved patient acceptance. The diversity of types enhances treatment scope and market expansion.
- For instance, MannKind’s inhalable insulin Afrezza® reaches peak plasma concentration in 13 minutes with 24% bioavailability relative to subcutaneous insulin, enabling rapid glycemic control.
By Application:
The inhalable biologics market by application covers respiratory diseases, oncology, autoimmune disorders, and others. Respiratory diseases dominate due to the high prevalence of asthma, COPD, and cystic fibrosis. Oncology represents a growing segment supported by clinical trials exploring pulmonary delivery of biologics. It is expanding into autoimmune conditions as researchers explore efficient drug targeting through inhalation. Broader applications reflect strong potential for improving disease management. This diversity ensures the market evolves into a versatile therapeutic platform.
- For instance, aerosolized liposomal 9-nitro camptothecin (9NC) achieved lung drug concentrations 4–10 times higher than plasma in a Phase I trial for lung cancer, supporting its recommendation for Phase II dosing at 13.3 µg/kg/day and demonstrating preliminary tumor responses.
By Distribution Channel:
The inhalable biologics market by distribution channel includes hospital pharmacies, retail pharmacies, and online platforms. Hospital pharmacies hold the largest share due to strong demand for biologics in critical care settings. Retail pharmacies support adoption through accessibility and widespread availability of inhalation devices. It is witnessing rising sales through online platforms as digital health adoption grows. Online channels expand access for patients seeking convenience and remote treatment options. This multi-channel distribution strengthens market reach and patient compliance.
Segmentations:
By Type:
- Monoclonal Antibodies
- Proteins
- Peptides
- Vaccines
By Application:
- Respiratory Diseases
- Oncology
- Autoimmune Disorders
- Others
By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Platforms
By Region:
- North America
- Europe
- UK
- France
- Germany
- Italy
- Spain
- Russia
- Belgium
- Netherlands
- Austria
- Sweden
- Poland
- Denmark
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Indonesia
- Vietnam
- Malaysia
- Philippines
- Taiwan
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Peru
- Chile
- Colombia
- Rest of Latin America
- Middle East
- UAE
- KSA
- Israel
- Turkey
- Iran
- Rest of Middle East
- Africa
- Egypt
- Nigeria
- Algeria
- Morocco
- Rest of Africa
Regional Analysis:
North America:
North America held 42% share of the inhalable biologics market in 2024, driven by robust R&D investments and advanced healthcare systems. The United States remains the central hub due to strong biopharma presence and favorable reimbursement structures. Canada supports growth through government-backed healthcare initiatives and rising adoption of biologics. It benefits from widespread use of digital inhalation devices that improve adherence and monitoring. Strong collaborations between biotech firms and device manufacturers further accelerate innovation. The region continues to lead with faster regulatory approvals and higher acceptance of novel therapies.
Europe:
Europe accounted for 31% share of the inhalable biologics market in 2024, supported by strict EU regulations promoting sustainable drug delivery solutions. Germany, France, and the United Kingdom drive growth with strong clinical research and manufacturing capabilities. Rising focus on non-invasive treatments supports adoption across respiratory and autoimmune conditions. It is strengthened by funding for precision medicine and digital health integration. Strong presence of multinational pharmaceutical companies creates a competitive landscape. Europe benefits from harmonized regulatory pathways that accelerate approval and commercialization of inhalable biologics.
Asia Pacific:
Asia Pacific represented 19% share of the inhalable biologics market in 2024, with rapid expansion fueled by large patient populations and rising healthcare investments. China, Japan, and India are leading growth through increased clinical trials and adoption of advanced biologics. Rising prevalence of respiratory disorders drives strong demand for inhalation-based treatments. It is supported by government healthcare reforms and growing pharmaceutical partnerships. Expanding access to affordable inhalation devices accelerates uptake across developing economies. Asia Pacific is positioned as the fastest-growing region due to its scale and evolving healthcare infrastructure.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- AbbVie
- Boehringer Ingelheim
- AstraZeneca
- CanSino Biologics
- F-Hoffman Roche
- Chiesi Pharmaceuticals
- Kamada Pharmaceuticals
- Merxin
- Teva Pharmaceutical
- Mannkind
- United Therapeutics
Competitive Analysis:
The inhalable biologics market is shaped by strong competition among global and regional pharmaceutical players. Key companies include AbbVie, Boehringer Ingelheim, AstraZeneca, CanSino Biologics, F. Hoffmann-La Roche, Chiesi Pharmaceuticals, Kamada Pharmaceuticals, and Merxin. These firms focus on advancing inhalation technologies, improving drug stability, and expanding product pipelines across respiratory and non-respiratory applications. It is supported by strategic partnerships, licensing deals, and growing investment in research and clinical trials. Companies compete through innovation in delivery devices such as dry powder inhalers and smart inhalation systems. Regional expansion strategies target emerging markets with rising healthcare infrastructure and patient demand. The competitive landscape reflects continuous efforts to combine biologic expertise with device innovation, strengthening long-term growth potential.
Recent Developments:
- In August 2025, AbbVie announced the acquisition of Gilgamesh Pharmaceuticals’ lead investigational therapy, bretisilocin (GM-2505), to expand its psychiatry pipeline.
- In August 2025, Boehringer Ingelheim formalized a partnership with AnGes to develop and manufacture next-generation hepatocyte growth factor gene therapy for peripheral arterial disease.
- In June 2025, AstraZeneca forged a partnership with CSPC Pharmaceutical, valued at up to $5.3 billion, to utilize CSPC’s AI platform for the development of oral drugs targeting chronic and immunological diseases.
Report Coverage:
The research report offers an in-depth analysis based on Type, Application, Distribution Channel and Region. It details leading Market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current Market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven Market expansion in recent years. The report also explores Market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on Market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the Market.
Future Outlook:
- The inhalable biologics market is expected to expand with broader therapeutic applications beyond respiratory diseases.
- Biopharma companies will increase investments in advanced particle engineering and formulation technologies.
- Smart inhalation devices integrated with digital health platforms will strengthen patient adherence and monitoring.
- Partnerships between pharmaceutical firms and device manufacturers will drive faster commercialization of innovative solutions.
- Regulatory agencies are likely to provide clearer guidance for biologics-device combination approvals.
- Emerging markets will witness rapid adoption supported by growing healthcare infrastructure and rising patient populations.
- Clinical trials targeting oncology, diabetes, and autoimmune disorders will expand the scope of inhalable biologics.
- Advances in cost-effective manufacturing will improve scalability and accessibility in both developed and developing regions.
- Personalized medicine approaches will leverage inhalable biologics for targeted and efficient patient care.
- Long-term, the market will evolve as a key alternative to injectable biologics, offering convenience and efficacy for chronic disease management.




