Market Overview:
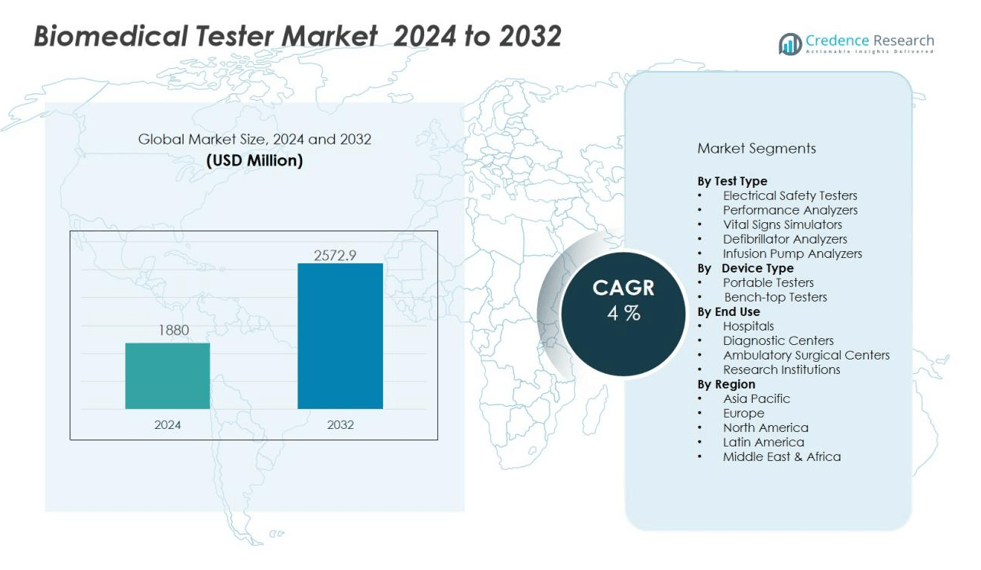
The biomedical tester market size was valued at USD 1880 million in 2024 and is anticipated to reach USD 2572.9 million by 2032, at a CAGR of 4 % during the forecast period (2024-2032).
| REPORT ATTRIBUTE |
DETAILS |
| Historical Period |
2020-2023 |
| Base Year |
2024 |
| Forecast Period |
2025-2032 |
| Medical Device Testing Market Size 2024 |
USD 1880 million |
| Medical Device Testing Market, CAGR |
4% |
| Medical Device Testing Market Size 2032 |
USD 2572.9 million |
Market growth is driven by multiple factors, including the increasing prevalence of chronic diseases, rising demand for point-of-care diagnostics, and the growing adoption of medical devices in hospitals, clinics, and research centers. Continuous innovation in biomedical testers, such as automation, wireless connectivity, and integration with cloud platforms, enhances efficiency and user convenience. Strict government regulations and safety standards mandate periodic performance testing, creating consistent demand for these devices across developed and developing economies.
Regionally, North America leads the biomedical tester market due to its advanced healthcare infrastructure, strong regulatory enforcement, and early adoption of innovative medical technologies. Europe holds a significant share, supported by robust government healthcare spending and compliance with stringent equipment standards. Asia-Pacific is poised to record the fastest growth, fueled by rapid healthcare expansion, rising patient volumes, and government initiatives in countries such as China, India, and Japan. Emerging markets in Latin America and the Middle East & Africa also present opportunities, backed by improving healthcare access and growing investments in medical equipment.
Market Insights:
- The biomedical tester market was valued at USD 1880 million in 2024 and is projected to reach USD 2572.9 million by 2032, reflecting steady growth driven by demand for reliable medical device validation.
- Rising prevalence of chronic diseases and increased adoption of point-of-care diagnostics continue to accelerate market expansion across hospitals, clinics, and research centers.
- Continuous innovation, including automation, wireless connectivity, and cloud-based integration, is transforming biomedical testers into highly efficient and user-friendly solutions.
- High costs of advanced testers and shortage of skilled professionals remain key challenges, especially in developing regions with budget constraints and complex regulatory requirements.
- North America holds 38 percent market share, Europe accounts for 30 percent, and Asia-Pacific captures 25 percent, with the latter emerging as the fastest-growing region due to expanding healthcare infrastructure and government initiatives.
Access crucial information at unmatched prices!
Request your sample report today & start making informed decisions powered by Credence Research Inc.!
Download Sample
Market Drivers:
Rising Need for Accurate Equipment Performance and Patient Safety:
The biomedical tester market is driven by the increasing importance of ensuring accuracy and reliability in medical devices. Hospitals and clinics rely on biomedical testers to validate performance and maintain compliance with safety standards. It plays a vital role in minimizing risks of device malfunction that could compromise patient outcomes. Growing awareness of patient safety has intensified demand across healthcare providers, accelerating market expansion.
- For instance, Fluke Biomedical’s ESA612 Electrical Safety Analyzer includes internal memory for 100 test records, enabling technicians to store and review extensive compliance data on‐site (100 records)
Regulatory Compliance and Standardization in Healthcare Systems:
Strict government regulations and international standards act as strong drivers for the biomedical tester market. Medical equipment manufacturers and healthcare providers must regularly test devices to meet compliance requirements. It ensures that biomedical testers remain indispensable tools for routine inspection and certification. Rising enforcement of quality control protocols across developed and emerging regions sustains consistent adoption.
- For instance, Mindray’s BeneView T1 patient monitor achieved FDA 510(k) clearance within standard regulatory timeframes, demonstrating successful compliance with IEC 60601-1 electrical safety standards and ANSI/AAMI requirements for multi-parameter patient monitoring devices.
Technological Advancements Enhancing Efficiency and Connectivity:
Continuous innovation in biomedical testers is shaping the growth trajectory of the market. The integration of wireless connectivity, automation features, and data management systems improves usability and reduces human error. It enables healthcare professionals to conduct faster, more accurate testing with seamless reporting capabilities. Demand for advanced, connected testers is increasing across both hospital and diagnostic settings.
Expanding Healthcare Infrastructure in Emerging Economies:
The rapid expansion of healthcare infrastructure in Asia-Pacific, Latin America, and parts of Africa significantly drives the biomedical tester market. Rising patient volumes and government investments in modern healthcare facilities create demand for reliable biomedical testing equipment. It aligns with the growing need to strengthen medical device quality and functionality in resource-limited settings. The shift toward improved healthcare access in these regions supports long-term growth opportunities.
Market Trends:
Integration of Digital Technologies and Smart Connectivity:
The biomedical tester market is witnessing a strong shift toward digitalization and connectivity-driven solutions. Manufacturers are focusing on developing testers with wireless communication, cloud integration, and automated data management features. It allows healthcare providers to streamline testing processes, ensure traceability, and improve compliance reporting. Growing adoption of Internet of Things (IoT) and artificial intelligence in testing platforms enhances diagnostic precision and operational efficiency. The demand for portable and handheld biomedical testers with advanced software interfaces is also rising, supporting point-of-care applications. This trend aligns with the broader digital transformation of healthcare infrastructure worldwide.
- For instance, Abbott’s i-STAT Alinity point-of-care analyzer delivers results for 17 analytes in under 10 minutes using a single cartridge
Growing Demand for Preventive Maintenance and Sustainability-Oriented Solutions:
Healthcare facilities are increasingly prioritizing preventive maintenance of medical equipment, fueling demand for biomedical testers designed for routine and predictive testing. The market is shifting toward sustainable designs with energy-efficient components and longer device lifecycles. It addresses the dual objective of reducing operational costs and meeting environmental goals in healthcare systems. The trend is further supported by rising awareness of eco-friendly medical technologies and stricter sustainability guidelines in developed regions. Outsourcing of biomedical testing services to specialized providers is also gaining momentum, offering cost efficiency and access to advanced expertise. This focus on sustainability and maintenance efficiency is reshaping long-term demand patterns across the global biomedical tester market.
- For instance, Abbott achieved a 7% reduction in Scope 1 and 2 emissions in 2023, complemented by energy and emission reduction projects that resulted in 28 million kWh annual energy savings.
Market Challenges Analysis:
High Cost of Advanced Testers and Limited Budget Allocation in Healthcare:
The biomedical tester market faces challenges due to the high cost of advanced testing equipment. Many healthcare providers, especially in developing regions, struggle to allocate sufficient budgets for acquiring and maintaining these devices. It creates a barrier for widespread adoption and limits accessibility in smaller hospitals and clinics. Frequent software upgrades and calibration needs further add to operational expenses. Price sensitivity in cost-constrained healthcare systems makes it difficult for manufacturers to penetrate emerging markets effectively.
Shortage of Skilled Professionals and Complex Regulatory Environment:
A significant challenge lies in the shortage of trained personnel capable of operating sophisticated biomedical testers. The complexity of modern devices requires specialized technical knowledge, which many facilities lack. It slows down adoption and raises the risk of underutilization of advanced equipment. At the same time, the biomedical tester market is influenced by strict regulatory frameworks that vary across regions, creating compliance burdens for manufacturers. Lengthy approval processes and evolving standards can delay product launches and increase development costs. These barriers collectively restrain growth potential, particularly for new entrants and smaller players.
Market Opportunities:
Rising Adoption of Portable and Point-of-Care Testing Devices:
The biomedical tester market presents strong opportunities through the growing demand for portable and point-of-care testing solutions. Healthcare facilities are increasingly shifting toward compact and user-friendly testers that can be deployed across multiple settings, including emergency units, ambulatory care, and remote healthcare facilities. It supports faster decision-making and enhances patient outcomes by ensuring timely equipment validation. The trend also benefits manufacturers investing in miniaturization and wireless capabilities to expand product portfolios. Growing emphasis on decentralized healthcare services further strengthens the need for portable biomedical testers.
Expansion in Emerging Economies and Integration with Smart Healthcare Systems:
Emerging economies offer significant growth opportunities driven by investments in healthcare infrastructure and increased patient volumes. Governments in Asia-Pacific, Latin America, and Africa are prioritizing modern diagnostic and monitoring capabilities, creating demand for advanced biomedical testers. It aligns with broader initiatives to strengthen healthcare quality and accessibility. Integration of testers with smart healthcare systems and IoT-enabled platforms opens new pathways for innovation and recurring revenue streams. The rising focus on predictive maintenance and digital healthcare ecosystems allows manufacturers to tap into long-term service opportunities while supporting sustainable growth in the biomedical tester market.
Market Segmentation Analysis:
By Test Type:
The biomedical tester market by test type includes electrical safety testers, performance analyzers, vital signs simulators, defibrillator analyzers, and infusion pump analyzers. Electrical safety testers dominate due to mandatory compliance requirements across hospitals and clinics. Performance analyzers also hold a significant share, driven by their role in ensuring accurate calibration of diagnostic and therapeutic devices. It benefits from the rising adoption of multi-parameter testers that combine efficiency and precision. Demand for specialized testers such as defibrillator and infusion pump analyzers continues to grow with the expansion of critical care services.
By Device Type:
By device type, the biomedical tester market is segmented into portable testers and bench-top testers. Portable testers are gaining strong momentum, supported by the demand for flexibility, ease of use, and suitability in point-of-care and emergency settings. Bench-top testers maintain relevance in large hospitals and laboratories where advanced capabilities and detailed reporting are required. It aligns with healthcare providers’ growing need for both mobile and stationary solutions to ensure comprehensive equipment testing.
- For instance, Gossen Metrawatt’s METRISO PRIME bench-top insulation tester achieves up to 100 GΩ maximum insulation resistance measurement in a single test.
By End User:
By end user, the biomedical tester market serves hospitals, diagnostic centers, ambulatory surgical centers, and research institutions. Hospitals remain the leading segment due to their high volume of medical devices and strict adherence to safety standards. Diagnostic centers are expanding usage with growing demand for accuracy and compliance. It finds increasing application in research institutions focusing on product development and validation of new biomedical devices.
- For instance, Siemens Healthineers’ Atellica Solution laboratory automation platform can process up to 440 tests per hour at full capacity. 440
Segmentations:
By Test Type:
- Electrical Safety Testers
- Performance Analyzers
- Vital Signs Simulators
- Defibrillator Analyzers
- Infusion Pump Analyzers
By Device Type:
- Portable Testers
- Bench-top Testers
By End User:
- Hospitals
- Diagnostic Centers
- Ambulatory Surgical Centers
- Research Institutions
By Region:
- North America
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- South-east Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of the Middle East and Africa
Regional Analysis:
North America:
North America holds 38 % market share in the biomedical tester market, supported by advanced healthcare infrastructure and early technology adoption. The United States leads demand with its strong base of hospitals, diagnostic centers, and regulatory enforcement for medical device safety. Canada contributes significantly with rising investments in healthcare modernization and compliance standards. It benefits from high awareness of patient safety and continuous integration of digital health solutions. Strong presence of leading manufacturers and service providers further strengthens regional dominance. Expansion of connected and portable testers ensures sustained growth across both large healthcare networks and smaller facilities.
Europe:
Europe accounts for 30% market share in the biomedical tester market, driven by strict compliance requirements and strong government funding for healthcare systems. Germany, the United Kingdom, and France lead demand with advanced hospitals and medical device manufacturers. It is supported by continuous technological upgrades in biomedical testing and integration with sustainable healthcare initiatives. Growing adoption of preventive maintenance practices across the region reinforces demand for testers. The presence of established players and research institutions also enhances product innovation and testing standards. Increasing collaborations between healthcare providers and technology companies further drive long-term opportunities.
Asia-Pacific:
Asia-Pacific captures 25 % market share in the biomedical tester market, with rapid expansion in healthcare infrastructure and rising patient volumes across China, India, and Japan. Government initiatives to strengthen diagnostic capabilities and improve hospital quality drive consistent investment in biomedical testing. It benefits from growing demand for affordable, portable, and connected testers suitable for diverse healthcare settings. Rising medical tourism and expanding private hospital networks add further momentum to regional growth. Local manufacturing capabilities and partnerships with global players support wider adoption of advanced technologies. Strong emphasis on improving healthcare access ensures Asia-Pacific remains the fastest-growing regional market.
Shape Your Report to Specific Countries or Regions & Enjoy 30% Off!
Key Player Analysis:
- Fluke
- Seaward Electronic Ltd.
- Datrend Systems Inc.
- Dynatech CBET
- Presto Group
- Southeastern Biomedical
- Illinois Tool Works Inc.
- BDC Laboratories
- NETECH CORPORATION
- Response Biomedical Corp.
Competitive Analysis:
The biomedical tester market is defined by a mix of global leaders and specialized regional players that compete through innovation, service quality, and regulatory compliance. Key companies such as Fluke, Seaward Electronic Ltd., Datrend Systems Inc., Dynatech CBET, Presto Group, Southeastern Biomedical, and Illinois Tool Works Inc. shape the competitive landscape with diverse product portfolios and strong distribution networks. It is characterized by high entry barriers, where technological expertise and adherence to strict standards determine market positioning. Leading firms focus on advanced features including automation, connectivity, and multi-parameter testing to differentiate offerings. Service providers strengthen competitiveness by offering calibration, maintenance, and technical support, ensuring long-term customer retention. It continues to evolve with growing emphasis on portable solutions and cloud-enabled platforms, creating opportunities for collaboration between medical device manufacturers and testing specialists. Competitive intensity remains strong, with established players leveraging scale advantages while niche firms compete on cost efficiency and specialized capabilities.
Recent Developments:
- In August 2025, Fluke Networks launched the Versiv Data Center Kits on August 18, 2025, providing advanced solutions for fiber and copper testing in data centers, significantly reducing testing time and improving network reliability.
- In May 2024, Dynatech CBET announced a strategic partnership with Sentera, becoming an authorized distributor of Sentera’s sensor and imaging solutions in the Middle East, focusing on agriculture and environmental monitoring.
- In March 2025, Presto Group acquired Velco Brandblusmateriaal B.V., a leading Dutch fire safety company, to expand its presence in the European market.
Market Concentration & Characteristics:
The biomedical tester market demonstrates moderate to high concentration, with a mix of established global players and regional manufacturers shaping competition. It is characterized by continuous innovation, strict regulatory compliance, and strong demand for accuracy in medical device validation. Large companies dominate with advanced product portfolios, extensive distribution networks, and integrated service offerings, while smaller firms compete by focusing on cost-effective and portable solutions. The market exhibits high entry barriers due to regulatory approvals, technological complexity, and capital requirements. It is further defined by recurring demand from healthcare providers driven by preventive maintenance and safety mandates. Growing emphasis on digital integration, wireless connectivity, and sustainability reflects its evolving characteristics and long-term development.
Report Coverage:
The research report offers an in-depth analysis based on Test Type, Device Type, End User and Region. It details leading Market players, providing an overview of their business, product offerings, investments, revenue streams, and key applications. Additionally, the report includes insights into the competitive environment, SWOT analysis, current Market trends, as well as the primary drivers and constraints. Furthermore, it discusses various factors that have driven Market expansion in recent years. The report also explores Market dynamics, regulatory scenarios, and technological advancements that are shaping the industry. It assesses the impact of external factors and global economic changes on Market growth. Lastly, it provides strategic recommendations for new entrants and established companies to navigate the complexities of the Market.
Future Outlook:
- The biomedical tester market will continue to expand with rising demand for accurate performance validation of medical devices across healthcare facilities.
- Integration of IoT-enabled platforms and cloud-based data management will enhance real-time monitoring and reporting capabilities.
- Portable and handheld biomedical testers will gain traction as healthcare systems prioritize flexibility and point-of-care applications.
- Strong regulatory requirements will sustain consistent demand for device testing and certification across developed and emerging regions.
- Investments in predictive maintenance solutions will create opportunities for recurring revenues and service-based models.
- Technological advancements, including automation and artificial intelligence, will improve efficiency and reduce human error in biomedical testing.
- Emerging economies will drive growth through healthcare infrastructure development and increased patient volumes.
- Sustainability and eco-friendly product design will shape innovation, aligning with global healthcare sustainability goals.
- Collaborations between medical device manufacturers and technology firms will accelerate product development and broaden adoption.
- Expanding digital healthcare ecosystems will position biomedical testers as integral tools supporting connected, compliant, and efficient healthcare delivery.




